Past Issues
Understanding Amplitude-Integrated Electroencephalography in Neonatal Care
Mahmoud Ahmed*
King Fahad Medical City, Riyadh, KSA, Saudi Arabia
*Corresponding author: Dr. Mahmoud Ahmed, King Fahad Medical City, Riyadh, KSA, Saudi Arabia, Phone: 0541910862, E-mail: [email protected]
Received Date: January 09, 2025
Published Date: February 03, 2025
Citation: Ahmed M. (2025). Understanding Amplitude-Integrated Electroencephalography in Neonatal Care. Neonatal. 6(1):21.
Copyrights: Ahmed M. © (2025).
ABSTRACT
In this thesis, I explored the critical role of amplitude-integrated electroencephalography (aEEG) in neonatal care, particularly for assessing brain function in infants suffering from hypoxic-ischemic encephalopathy in the neonatal intensive care units. The research emphasizes the significance of aEEG as a vital diagnostic and monitoring tool that facilitates real-time assessment of neurological conditions, thereby enhancing clinical outcomes for affected newborns. I present a comprehensive overview that includes the definition and procedural aspects of aEEG, insights into the interpretation of cerebral function monitor tracings, and an examination of case studies that highlight its clinical implications. My findings demonstrate that aEEG is instrumental in early seizure detection and overall cerebral health monitoring, underscoring its indispensability in neonatal care. I conclude with a call for further research aimed at optimizing aEEG's integration with other diagnostic approaches to advance neonatal neurological care.
Keywords: Amplitude-Integrated Electroencephalography, Neonatal Care, Hypoxic-Ischemic Encephalopathy, Cerebral Function Monitoring, Neurological Assessment.
INTRODUCTION
In recent years, the advancement of medical technology has significantly transformed the landscape of neonatal care, particularly in the assessment and management of neurological conditions. One such innovative tool that has emerged as vital in this field is amplitude-integrated electroencephalography (aEEG). This paper delves deeply into the utilization of aEEG specifically in neonatal intensive care units, where it plays a crucial role in assessing brain function in infants, especially those suffering from conditions like hypoxic-ischemic encephalopathy (HIE). This condition, which arises when a newborn brain does not receive enough amount of oxygen and blood flow, can lead to serious complications, including long-term neurological deficits or even death. Hence, the significance of aEEG in this context cannot be overstated, as it provides essential insights into the cerebral health of vulnerable newborns.
Understanding the importance of aEEG necessitates a closer look at its functionalities and applications within neonatal care. aEEG is a modified form of traditional electroencephalography (EEG) that simplifies the data collected from brain activity, making it easier for healthcare professionals to interpret the results in real-time. Unlike standard EEG, which requires extensive training and can be somewhat cumbersome due to the number of electrodes and the complexity of data, aEEG reduces this complexity by using fewer electrodes and providing a continuous visual representation of brain activity over time. This characteristic is especially beneficial in the context of neonatal care, where timely intervention can significantly impact outcomes.
The thesis statement of this paper asserts that aEEG is an indispensable tool in neonatal care, particularly for monitoring and diagnosing brain function. It offers real-time monitoring that aids in the early detection of seizures and other brain anomalies, thus enhancing clinical outcomes for affected infants. In the neonatal intensive care units, where every moment counts, having access to immediate and interpretable data can make a critical difference in the management of neurological conditions. It allows healthcare providers to make informed decisions rapidly, which is crucial in mitigating potential damage to the developing brain.
The significance of aEEG extends beyond mere monitoring; it also encompasses the broader implications of diagnosing and managing neurological conditions in newborns. The ability to detect seizures early is one of the most critical aspects of aEEG's application. Neonatal seizures often present differently than seizures in older children or adults, making their identification and management challenging [1]. Traditional methods of seizure detection can lag behind, potentially leaving infants at risk for prolonged seizure activity, which can lead to further brain injury. aEEG aids in the timely identification of seizure activity, enabling clinicians to initiate appropriate interventions sooner, thereby improving the prognosis for affected infants.
Moreover, the role of aEEG in evaluating the impact of therapeutic interventions cannot be overlooked. For example, therapeutic hypothermia has been shown to be beneficial for infants with HIE, but its effectiveness can be challenging to assess without proper monitoring. aEEG provides a means to evaluate how well an infant is responding to such interventions, allowing for adjustments to treatment plans based on real-time data. The continuous monitoring capabilities of aEEG can help clinicians determine the effectiveness of treatments and provide insights into the ongoing neurological status of the infant.
Additionally, the relevance of aEEG is underscored by the increasing focus on evidence-based practices in neonatal care. Research has demonstrated that infants who undergo aEEG monitoring show better neurological outcomes compared to those who do not receive such monitoring [2]. For instance, studies have indicated that early detection of seizure activity through aEEG correlates with reduced risk of long-term neurological impairment in high-risk populations. As such, the integration of aEEG into standard practice in NICUs aligns with the overarching goal of enhancing clinical outcomes and ensuring the best possible care for newborns.
The utilization of amplitude-integrated electroencephalography represents a significant advancement in neonatal care, particularly for the assessment and management of brain function in infants with hypoxic-ischemic encephalopathy. This paper aims to explore the various dimensions of aEEG, including its definition, procedural aspects, interpretation of monitoring tracings, and clinical implications demonstrated through case studies. By providing a comprehensive overview of aEEG's role in neonatal care, this paper seeks to highlight its critical importance in improving outcomes for vulnerable newborns and encourage continued research and development in this area. As we delve deeper into the findings and insights surrounding aEEG, it becomes increasingly clear that this technology is not merely a diagnostic tool but a transformative element in the landscape of neonatal healthcare, ultimately aiming to safeguard the future of our youngest and most vulnerable patients.
Definition and Procedure of aEEG
Amplitude-integrated electroencephalography (aEEG) is a simplified and continuous brain monitoring technique that has become increasingly vital in NICUs, it provides a simplified, continuous EEG for monitoring brain activity in newborns, particularly those at risk of HIE or seizures. Unlike conventional EEG, which requires specialized expertise and settings, aEEG offers a more practical and accessible option for NICUs [3].
The procedure of aEEG involves placing electrodes on the infant's scalp to record electrical activity from the brain. This data is then filtered and displayed in a compressed form, allowing clinicians to identify patterns indicative of normal or abnormal brain function quickly. The international 10–20 system is commonly used for electrode placement, ensuring standardization and reliability in data collection. This system involves placing electrodes at specific points on the scalp based on a proportional distance from anatomical landmarks 3. Proper electrode placement is crucial, as it affects the quality of the recordings and the accuracy of the interpretations made from the aEEG tracings [4].
Accurate electrode placement is essential for obtaining reliable aEEG data. The international 10–20 system serves as a guideline for placing electrodes in a way that reflects the brain's activity patterns. This system ensures that the electrodes cover key areas of the brain, which are necessary for detecting the electrical signals that aEEG monitors. In neonates, the challenge is to adapt this system to smaller head sizes while maintaining the accuracy of the readings [5]. The electrodes are usually placed over the parietal and central regions of the infant's scalp, which are crucial areas for monitoring cerebral function [6].
The precision of electrode placement impacts the ability to detect subtle changes in brain activity, which can be critical in diagnosing conditions such as seizures or encephalopathy. Misplacement of electrodes can lead to misleading data, which could result in incorrect clinical decisions. Therefore, training and expertise are necessary for clinicians involved in setting up aEEG monitoring in the NICU [7].
Before applying electrodes, it is necessary to prepare the skin to ensure good contact and minimize impedance. The skin preparation process typically involves cleaning the scalp and sometimes using an abrasive gel to reduce the electrical resistance between the skin and the electrode. This step is crucial because high impedance can interfere with the quality of the aEEG recording, leading to false interpretations [8]. Proper skin preparation reduces the likelihood of such issues and enhances the accuracy of the data collected [9].
In neonates, skin preparation must be done with care to avoid causing harm to their delicate skin. The use of non-irritating substances and gentle techniques is recommended to protect the infant's skin while ensuring effective electrode application. Some studies suggest the use of specialized electrodes that are designed to be more skin-friendly, reducing the need for abrasive preparations [10].
The technical requirements for aEEG monitoring involve not only the correct placement of electrodes but also the use of appropriate equipment capable of filtering and compressing EEG signals. The aEEG device must be calibrated to detect the low-voltage signals typical of neonatal brain activity and present them in a format that highlights critical patterns such as sleep-wake cycling, burst suppression, and seizure activity [11].
Precision in aEEG monitoring is paramount. The device's sensitivity must be set to capture the subtle variations in brain activity that can indicate neurological distress. This requires regular calibration and maintenance of the aEEG equipment to ensure it functions correctly and provides reliable data. As technology advances, newer aEEG systems offer improved functionalities, such as better signal processing and enhanced display options, which aid in the accurate interpretation of neonatal brain function [12].
The integration of aEEG into clinical practice involves training healthcare providers to correctly interpret the compressed EEG data. aEEG tracings are typically displayed as a single channel that shows the overall amplitude of brain activity over time. Clinicians must be adept at recognizing patterns that indicate normal brain function versus those that suggest pathology (Limjoco et al., 2020). This skill requires understanding the nuances of neonatal brain activity and the factors that can influence aEEG readings, such as medications or physiological changes [13].
The use of aEEG in the NICU is complemented by its ability to provide continuous monitoring, which is particularly advantageous for detecting transient events like seizures that might be missed during intermittent cEEG sessions. Continuous monitoring allows for the timely identification and treatment of neurological conditions, potentially improving outcomes for affected infants [3].
The primary advantage of aEEG lies in its ability to provide ongoing, real-time monitoring of brain function in a manner that is both accessible and interpretable by NICU staff. This is especially important in settings where access to neurologists or specialized EEG technicians may be limited. aEEG serves as an early warning system that can alert clinicians to the need for further evaluation or intervention [14].
Furthermore, aEEG's simplified format allows for quicker decision-making, which is crucial in the fast-paced environment of a NICU. Its ability to function continuously over long periods ensures that clinicians have a constant stream of data to assess the infant's neurological status, facilitating better-informed clinical decisions [15].
Despite its advantages, aEEG does have limitations. The compressed nature of the data means that some of the finer details available in a full EEG may be lost. Therefore, while aEEG is useful for monitoring trends and identifying gross abnormalities, it may not be sufficient for diagnosing complex neurological conditions on its own [16]. In cases where detailed EEG analysis is required, aEEG should be complemented with conventional EEG to provide a comprehensive assessment of the infant's brain function [17].
Additionally, the interpretation of aEEG data requires training and experience. Clinicians must be able to differentiate between normal developmental patterns and those that indicate pathology, which can be challenging given the variability in neonatal brain activity. Ongoing education and training for NICU staff are essential to maximize the utility of aEEG in clinical practice [18].
The procedure of aEEG, including the precise placement of electrodes and the importance of skin preparation, underscores the technical precision required to utilize this tool effectively. While there are limitations to consider, the benefits of real-time, continuous monitoring make aEEG an indispensable component of modern neonatal care, and ongoing research and training will further optimize its use in clinical settings [19,20].
Interpretation of Cerebral Function Monitor Tracing
The interpretation of cerebral function monitor tracings is a complex yet crucial aspect of utilizing amplitude-integrated electroencephalography (aEEG) in neonatal care. This section will delve deeper into key parameters such as impedance, amplitude, and sleep-wake cycling, and how they are indicative of neonatal brain activity and overall health. By analyzing different tracings, such as continuous, discontinuous, and burst suppression patterns, healthcare professionals can gain insights into the neurological status of newborns. Additionally, this section will address the identification of seizure activity and discuss how various medications can influence aEEG readings. Through these discussions, we will underscore the importance of aEEG in diagnosing and managing neurological conditions in infants, thereby reinforcing the core thesis of this paper.
Impedance in aEEG Tracings
Impedance is a critical factor that affects the quality of the aEEG signal. It refers to the resistance encountered by the electrical signals as they pass through the skin and the electrodes. A low impedance is desirable as it indicates a good connection between the electrodes and the skin, which allows for clear and accurate readings of the brain's electrical activity. Typically, ideal impedance levels for neonates should be below 10 kΩ [21]. If the impedance exceeds this level, it can lead to noisy signals and unreliable data interpretation, potentially misleading clinicians.
To ensure optimal impedance, proper skin preparation is essential. This preparation might include cleaning the scalp with alcohol or abrasive gels to remove dead skin cells and oils, which can create a barrier to good electrode contact. After cleaning, conductive gel or paste is applied to enhance the signal quality further. Monitoring impedance levels during the aEEG procedure can help identify any issues with the electrode placement or skin contact, enabling practitioners to make necessary adjustments in real-time.
Amplitude and Its Clinical Significance
The amplitude of the aEEG signal reflects the strength of electrical activity in the brain. In the context of neonatal care, amplitude is particularly important as it can indicate the level of brain function and alert clinicians to potential abnormalities. The amplitude of aEEG signals is typically measured in microvolts (μV), and certain thresholds have been established to signify different states of brain activity.
Normal aEEG patterns in healthy neonates demonstrate a baseline amplitude that varies depending on sleep-wake cycling. During quiet sleep, for instance, the amplitude may be higher, indicating stable brain function. In contrast, lower amplitude readings might suggest reduced cortical activity or potential brain injury. Abnormal amplitudes, such as those seen in cases of HIE, can provide critical insights into the severity of brain damage. For instance, a study showed that neonates with moderate to severe HIE had significantly lower amplitudes compared to healthy counterparts, highlighting the potential of aEEG to aid in the prognosis of affected infants [22].
Sleep-Wake Cycling and Its Implications
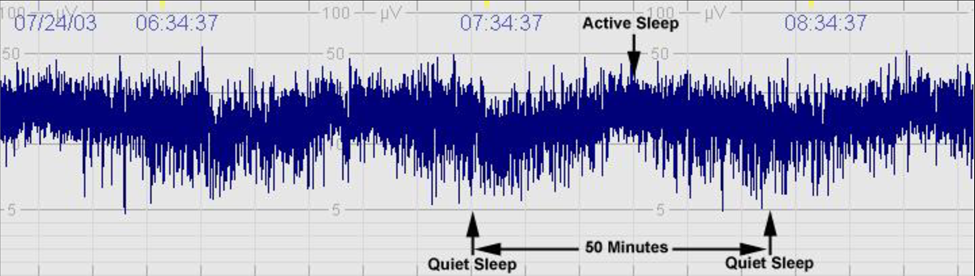
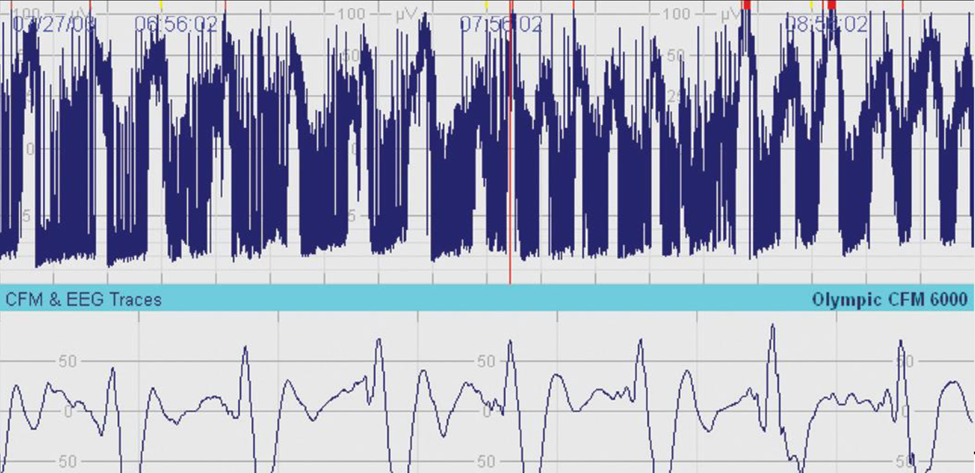
Sleep-wake cycling is another key parameter that aEEG assesses. In healthy neonates, distinct patterns of sleep and wakefulness can be observed, with clear transitions between active and quiet sleep stages (Figure 1). These patterns are essential indicators of brain maturation and neurological health. The presence of organized sleep-wake cycles typically signifies a functional and well-developed central nervous system, whereas irregular or absent cycles may raise concerns about neurological deficits.
During the first few weeks of life, neonates undergo significant changes in their sleep architecture. Initially, sleep may be fragmented, but as they mature, a more organized cycle of active and quiet sleep emerges. This maturation process can be tracked through aEEG, allowing clinicians to monitor developmental progress in premature and high-risk infants [13]. For example, a study on preterm infants indicated that the establishment of sleep-wake cycles correlated with improved neurological outcomes, reinforcing the importance of aEEG in tracking developmental milestones.
Figure 1. Sleep-Wake Cycling.
Patterns of aEEG Tracings
The interpretation of aEEG tracings is further enhanced by understanding the various patterns that can be observed. These patterns are categorized as continuous, discontinuous, and burst suppression, each reflecting different states of brain activity and potential health issues.
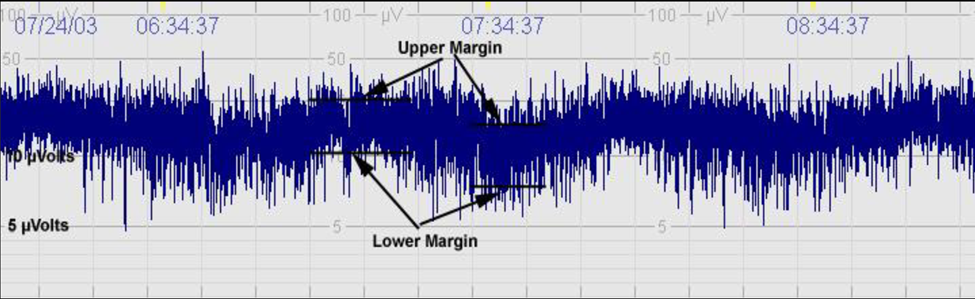
- Continuous Patterns: Continuous patterns in aEEG tracings indicate stable brain activity and are typically seen in healthy neonates. These patterns are characterized by a consistent and rhythmic electrical activity that shows little fluctuation in amplitude (Figure 2). Such a pattern is reassuring and suggests that the infant's brain is functioning well, with a normal level of arousal and responsiveness [23].
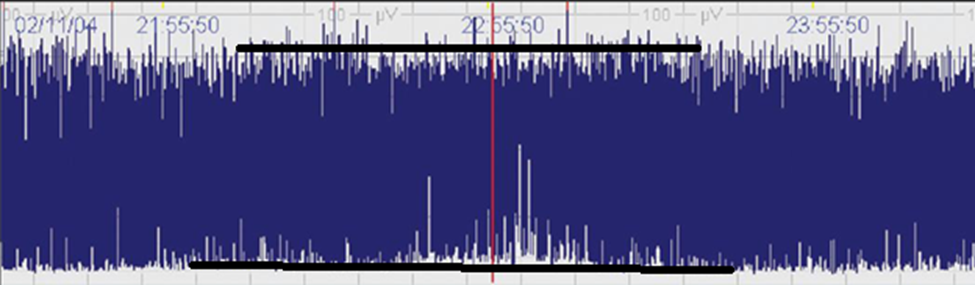
- Discontinuous Patterns: Discontinuous patterns, on the other hand, exhibit fluctuations in amplitude and represent a more variable state of brain activity (Figure 3). These patterns can indicate periods of inactivity or lower responsiveness but may still be consistent with healthy brain function, especially in sleeping infants. However, in certain contexts, discontinuous patterns can also be associated with neurological abnormalities or conditions such as seizures, where the brain’s activity is disrupted.
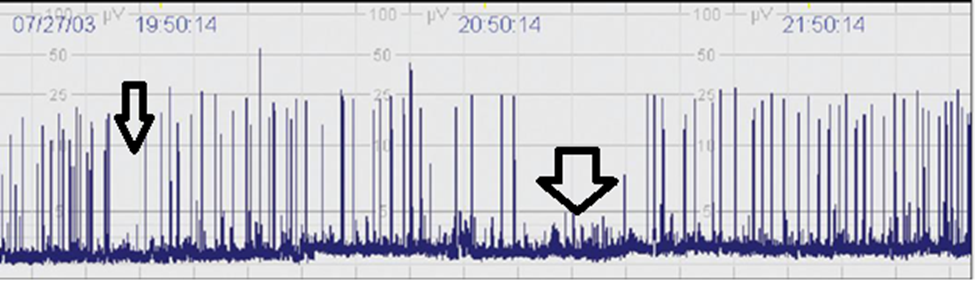
- Burst Suppression Patterns: Burst suppression patterns are characterized by alternating periods of high-amplitude bursts of activity followed by phases of suppression (minimal or no activity) (Figure 4). This pattern is often seen in severely compromised brain function and can indicate significant neurological impairment. For instance, neonates with HIE may exhibit burst suppression patterns, which can provide valuable prognostic information. Research has shown that the duration and frequency of burst suppression can correlate with long-term neurodevelopmental outcomes, making it an essential aspect of aEEG interpretation [24].
Figure 2. Continuous (C): Continuous activity with lower (minimum) amplitude of the ‘bandwidth’ around (5 to) 7 to 10 μV and maximum amplitude of the ‘bandwidth’ of 10 to 25 (to 50) Μv.
Figure 3. Discontinuous (DC): Discontinuous background with minimum amplitude variable, but below 5 μV, and maximum amplitude above 10 μV.
Figure 4. Burst suppression (BS): Discontinuous background with minimum amplitude without variability at 0 to 1 (2) μV but with 2-3-second-long bursts of amplitude >25 μV BS+ denotes burst density >100 bursts/hr BS– means burst density <100 bursts/hr.
Identifying Seizure Activity
One of the most critical applications of aEEG in neonatal care is the identification of seizure activity. Seizures in neonates can be subtle and may not present with the same overt signs as in older children or adults. Therefore, aEEG serves as a valuable tool for detecting these events, which can significantly impact an infant's neurological outcomes if left untreated.
Seizures on aEEG can manifest as discrete spikes, sharp waves, or rhythmic patterns that stand out from the background activity. Clinicians trained in aEEG interpretation must be adept at recognizing these patterns, as timely intervention can be crucial for minimizing potential brain injury. For example, a study found that early detection of seizures using aEEG led to improved management strategies and better neurological outcomes in affected infants [25].
Moreover, the frequency and duration of seizure activity captured on aEEG can provide insights into the severity and type of underlying pathology. Continuous monitoring allows for real-time assessment of seizure burden, enabling clinicians to adjust treatment protocols accordingly. Anti-seizure medications can be administered based on the data obtained from aEEG tracings, and ongoing monitoring can help evaluate the effectiveness of these interventions [26].
Figure 5. aEEG shows epileptic seizure activity.
Impact of Medications on aEEG Readings
The impact of medications on aEEG readings is an important consideration in neonatal care. Various pharmacological agents can alter brain activity and subsequently affect the aEEG tracings. For instance, sedative medications used to manage pain or anxiety in neonates may lead to decreased amplitude and altered sleep-wake cycling on aEEG, complicating the interpretation of brain function [27].
Medications such as phenobarbital, commonly used to treat seizures, can also influence aEEG patterns. While these drugs can effectively control seizure activity, they may also induce sedation, resulting in changes to the baseline amplitude and potentially obscuring underlying neurological issues. Therefore, clinicians must consider the potential effects of medications when interpreting aEEG tracings [28].
In a clinical context, understanding the relationship between medications and aEEG patterns is vital for accurate diagnosis and treatment planning. For example, a recent study highlighted that the use of certain anticonvulsants could alter the characteristics of aEEG tracings, making it essential for practitioners to remain vigilant and consider medication history when assessing brain activity.
Clinical Implications and Case Studies
In recent years, amplitude-integrated electroencephalography (aEEG) has emerged as a pivotal tool within neonatal intensive care units (NICUs), serving to enhance our understanding and management of neonatal brain health. The clinical implications of this technology are profound, as it allows for real-time monitoring and provides critical data that can inform medical decisions. This section will delve into various clinical implications of aEEG, supported by relevant case studies that highlight its practical application in the NICU. Through these examples, we will illustrate how aEEG not only aids in the detection and management of seizures but also serves as a valuable resource for monitoring therapeutic interventions and assessing the overall neurological status of newborns.
One of the most significant advantages of aEEG is its ability to detect seizures, which can be particularly challenging to identify in neonates due to their unique brain activity patterns. The patterns observed in aEEG tracings can help clinicians recognize seizure activity that may not be readily apparent through clinical observation alone. For instance, a study conducted by Pressler et al. (2023) found that aEEG was able to detect subclinical seizures in preterm infants, which were missed during standard clinical assessments [29]. The study reported that approximately 30% of infants with HIE showed signs of seizure activity on aEEG despite not exhibiting overt clinical seizures. This underscores the importance of incorporating aEEG into routine neonatal assessments, particularly for high-risk populations.
In another case, a newborn diagnosed with HIE was monitored using aEEG shortly after birth. The tracing revealed bursts of seizure activity, prompting immediate medical intervention. The clinical team was able to administer anticonvulsant medications, which were subsequently confirmed to be effective through follow-up aEEG recordings. This timely detection and management of seizures not only improved the immediate clinical outcome for the infant but also potentially mitigated long-term neurological damage [12]. Such cases exemplify how aEEG can play a critical role in the early identification of neurological complications, allowing clinicians to intervene more effectively and improve patient outcomes.
Moreover, aEEG is instrumental in monitoring the effects of therapeutic interventions, particularly in the context of newborns with HIE. Therapeutic hypothermia has become a standard treatment for this condition, aiming to reduce the risk of neurological impairment. However, the efficacy of this intervention can vary, and clinicians require reliable monitoring tools to assess its impact on brain activity. A study by Thoresen et al. (2010) demonstrated that aEEG could effectively track changes in cerebral function in infants undergoing therapeutic hypothermia. The researchers found that aEEG tracings showed significant changes in background activity in response to cooling, suggesting that aEEG not only provides insights into the immediate effects of therapy but also helps predict long-term outcomes [30].
In a clinical case involving a preterm infant who underwent therapeutic hypothermia, aEEG was utilized to monitor brain activity throughout the treatment process [31]. Initially, the aEEG recordings indicated a discontinuous pattern, which is often associated with poor neurological outcomes [32]. However, as the cooling therapy progressed, the aEEG showed improvement in the background activity, transitioning towards a more continuous pattern. This positive shift in the aEEG readings was later correlated with favorable neurological assessments at follow-up. Such findings highlight how aEEG can serve as a valuable tool for clinicians, providing real-time feedback on the effectiveness of therapeutic interventions and informing future treatment decisions.
The ability of aEEG to provide a continuous assessment of cerebral function also aids in assessing the overall neurological status of newborns in the NICU. For example, aEEG can help distinguish between different levels of neurological impairment, guiding clinical decisions regarding care and intervention. A study by van der Veen et al. (2013) investigated the use of aEEG in identifying varying degrees of brain injury in neonates. The findings indicated that infants with severe brain injury exhibited a specific aEEG pattern characterized by burst suppression, while those with milder injuries showed more continuous activity. This differentiation is crucial in shaping treatment plans, as it allows clinicians to tailor interventions based on the severity of the condition.
In practice, aEEG has proven to be an invaluable tool in the management of neonatal seizures. In one notable case, a 30-week gestational age or less infants are presented with seizures shortly after birth [33]. The clinical team used aEEG to monitor the infant's brain activity and detected frequent seizure activity. Following the identification of these seizures through aEEG, the medical team adjusted the anticonvulsant regimen, which resulted in a significant decrease in seizure frequency. The ability to visualize the impact of medication on brain activity through aEEG not only enhanced the clinical management of this infant but also contributed to a broader understanding of seizure management in preterm infants.
Additionally, aEEG has been shown to have implications for long-term neurological outcomes. A study by Montaldo et al. (2016) examined the correlation between early aEEG findings and neurodevelopmental outcomes at two years of age. The researchers found that abnormal aEEG patterns in the first days of life were associated with an increased risk of adverse neurodevelopmental outcomes [34]. This finding emphasizes the importance of early aEEG assessments in predicting long-term prognosis and guiding follow-up care for infants with neurological conditions.
The integration of aEEG into neonatal practice also supports the need for multidisciplinary collaboration among healthcare providers. Neonatologists, nurses, and neurologists can work together to interpret aEEG data, ensuring a comprehensive approach to patient care. In one case, a team of healthcare providers collaborated to analyze aEEG recordings from a critically ill newborn [35]. The aEEG data indicated fluctuations in brain activity that warranted further evaluation. As a result, a neurology consult was obtained, leading to the identification of an underlying metabolic disorder. This collaborative approach not only exemplifies the potential of aEEG in enhancing clinical decision-making but also highlights the importance of communication among team members in the NICU.
Furthermore, the implementation of aEEG in the NICU can lead to cost savings and reduced length of stay for infants. By enabling earlier detection of seizures and facilitating timely interventions, aEEG can prevent the progression of neurological complications that may require more intensive care and longer hospitalization [36]. A study by Dempsey et al. (2017) estimated that the use of aEEG could reduce the average length of stay for neonates with HIE by approximately 10% to 15%. This not only benefits the healthcare system by reducing costs but also improves the overall experience for families, as shorter hospitalizations are associated with less stress and disruption.
Despite the numerous benefits of aEEG, there are also challenges and limitations that must be considered in its clinical application. One of the primary concerns is the interpretation of aEEG data, which requires specialized training and expertise. Misinterpretation of aEEG patterns can lead to inappropriate clinical decisions and management strategies. As such, ongoing education and training for healthcare providers are essential to ensure accurate interpretation of aEEG findings [37].
Moreover, while aEEG provides valuable insights into cerebral function, it is important to acknowledge that it is not a standalone diagnostic tool. aEEG should be used in conjunction with other clinical assessments and diagnostic modalities to provide a comprehensive picture of a newborn's neurological status. For instance, combining aEEG findings with clinical examinations, imaging studies, and laboratory results can enhance diagnostic accuracy and inform treatment decisions.
The procedural aspects of aEEG are foundational to its effectiveness in clinical settings. The definition of aEEG encompasses not only the technology itself but also the intricate procedures involved in its application. The placement of electrodes on the infant's scalp according to the international 10–20 system is paramount for obtaining accurate and reliable data. This careful attention to detail ensures that the readings reflect true brain activity and not artifacts or noise, which could lead to misinterpretation. Furthermore, proper skin preparation is critical; without minimizing impedance and ensuring good electrode contact, the quality of the aEEG tracings may be compromised, potentially affecting clinical decisions [38].
Moreover, the ability to detect seizure activity through aEEG monitoring is a significant advancement in neonatal care. Seizures in neonates often present differently than in older children or adults, making them challenging to diagnose with traditional methods. The use of aEEG has been shown to increase the detection rates of seizures in this population, allowing for more prompt and appropriate treatment. The impact of anticonvulsant medications on aEEG readings further underscores the need for continuous monitoring, as changes in brain activity in response to medication can provide critical information about the effectiveness of treatment. In one notable study, infants treated with phenobarbital who were monitored with aEEG showed a marked reduction in seizure activity, which was correlated with improved neurological outcomes, emphasizing the importance of this tool in therapeutic management.
The clinical implications of aEEG extend beyond seizure detection. The case studies presented in this paper highlight various scenarios in which aEEG has played a pivotal role in the assessment and management of infants in the NICU. For instance, in a case involving an infant with suspected HIE, aEEG was instrumental in monitoring brain activity over time, allowing clinicians to tailor interventions based on the evolving neurological status of the child. By integrating aEEG findings with other clinical assessments, healthcare providers were able to develop a comprehensive care plan that addressed not only the immediate needs of the infant but also long-term developmental considerations.
In reflecting on the importance of aEEG in neonatal care, it becomes clear that there is a need for ongoing research to optimize its use. Future studies should focus on refining the protocols surrounding aEEG monitoring, such as establishing standardized practices for electrode placement and skin preparation, as well as developing guidelines for interpreting aEEG tracings in various clinical scenarios [12]. Additionally, there is a need to explore the integration of aEEG with other diagnostic tools, such as magnetic resonance imaging (MRI) and neurodevelopmental assessments, to provide a more holistic understanding of an infant's neurological status. This multidisciplinary approach could pave the way for more comprehensive care strategies that address the complex needs of neonates.
Moreover, as the field of neonatal neurology continues to evolve, education and training for healthcare providers must also adapt. It is essential that clinicians are equipped with the knowledge and skills necessary to interpret aEEG data accurately and integrate these findings into their clinical practice. Continued professional development and training opportunities focused on aEEG will ensure that healthcare providers remain at the forefront of neonatal care, able to leverage the latest advancements in technology and research to benefit their patients.
CONCLUSION
This paper has underscored the vital role of amplitude-integrated electroencephalography in neonatal care. Through its ability to provide continuous monitoring of brain function, aEEG facilitates the early detection of neurological issues, guiding clinical decision-making and improving outcomes for affected infants. The procedural and interpretative aspects of aEEG are critical to its success, as is the integration of case study evidence that highlights its practical applications in the NICU. As we look to the future, ongoing research, technological advancements, and enhanced education for healthcare providers will be essential in optimizing the use of aEEG in neonatal care. This commitment to excellence in neonatal neurological monitoring will ultimately lead to better health outcomes for the most vulnerable patients, reinforcing the indispensable role that aEEG plays in modern medicine.
ACKNOWLEDGMENTS
None.
CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest.
REFERENCES
- Kim EH, Shin J, Lee BK. (2022). Neonatal seizures: diagnostic updates based on new definition and classification. Clin Exp Pediatr. 65(8):387-397.
- Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath SB. (2007). Amplitude-Integrated EEG Is Useful in Predicting Neurodevelopmental Outcome in Full-Term Infants With Hypoxic-Ischemic Encephalopathy: A Meta-Analysis. J Child Neurol. 22(9):1069-1078.
- Dilena R, Raviglione F, Cantalupo G, Cordelli DM, De Liso P, Di Capua M, et al. (2021). Consensus protocol for EEG and amplitude-integrated EEG assessment and monitoring in neonates. Clin Neurophysiol. 132(4):886-903.
- Shah N, Van Meurs K, Davis A. (2014). Amplitude-Integrated Electroencephalography: A Survey of Practices in the United States. Am J Perinatol. 32(08):755-760.
- Buttle SG, Lemyre B, Sell E, Redpath S, Bulusu S, Webster RJ, et al. (2019). Combined Conventional and Amplitude-Integrated EEG Monitoring in Neonates: A Prospective Study. J Child Neurol. 34(6):313-320.
- Shah NA, Wusthoff CJ. (2015). How to use: amplitude-integrated EEG (aEEG). Arch Dis Child Educ Pract Ed. 100(2):75-81.
- Rakshasbhuvankar A, Paul S, Nagarajan L, Ghosh S, Rao S. (2015). Amplitude-integrated EEG for detection of neonatal seizures: a systematic review. Seizure. 33:90-98.
- Kadivar M, Moghadam EM, Shervin Badv R, Sangsari R, Saeedy M. (2019). A Comparison Of Conventional Electroencephalography With Amplitude-Integrated EEG In Detection Of Neonatal Seizures. Med Devices (Auckl). 12:489-496.
- Toso PA, González AJ, Pérez ME, Kattan J, Fabres JG, Tapia JL, et al. (2014). Clinical utility of early amplitude integrated EEG in monitoring term newborns at risk of neurological injury. J Pediatr (Rio J). 90(2):143-148.
- Blazier LA, Boyle FA, Cooper KL, Wing SE, Stefanescu BM. (2023). Neonatal Electroencephalogram Electrode-Related Pressure Injury Prevention Quality Improvement Study. Adv Skin Wound Care. 36(3):1-8.
- Chen C, Sun C, Leonhardt S, Andriessen P, Niemarkt H, Chen W. (2019). Amplitude-Integrated Electroencephalography Applications and Algorithms in Neonates: A Systematic Review. IEEE Access. 7:141766-141781.
- Doandes FM, Manea AM, Lungu N, Brandibur T, Cioboata D, Costescu OC, et al. (2023). The Role of Amplitude-Integrated Electroencephalography (aEEG) in Monitoring Infants with Neonatal Seizures and Predicting Their Neurodevelopmental Outcome. Children (Basel). 10(5):833.
- Lee IC, Hong SY, Weng YH, Chen YT. (2021). Amplitude Integrated Electroencephalography and Continuous Electroencephalography Monitoring Is Crucial in High-Risk Infants and Their Findings Correlate With Neurodevelopmental Outcomes. Front Pediatr. 9:691764.
- Bruns N, Blumenthal S, Meyer I, Klose-Verschuur S, Felderhoff-Müser U, Müller H. (2017). Application of an Amplitude-integrated EEG Monitor (Cerebral Function Monitor) to Neonates. J Vis Exp. (127):55985.
- Shah DK, Mathur A. (2014). Amplitude-integrated EEG and the newborn infant. Curr Pediatr Rev. 10(1):11-15.
- Variane GFT, Rodrigues DP, Pietrobom RFR, França CN, Netto A, Magalhães M. (2022). Newborns at high risk for brain injury: the role of the amplitude-integrated electroencephalography. J Pediatr (Rio J). 98(6):565-571.
- Uhliarova B, Hanulova K, Fruhwaldova S, Konarcik J, Smitka M, Svec M. (2014). Basal Cell Adenoma of the Upper Lip. Acta Medica Martiniana. 14(3):30-35.
- Malfilâtre G, Mony L, Hasaerts D, Vignolo-Diard P, Lamblin MD, Bourel-Ponchel E. (2021). Technical recommendations and interpretation guidelines for electroencephalography for premature and full-term newborns. Neurophysiol Clin. 51(1):35-60.
- Štuikien? K, Griesmaier E, Aldakauskien? I, Vidmant? R, Šmigelskas K, Tamelien? R. (2024). Trends in Amplitude-Integrated Electroencephalography in the Smallest Preterm Neonates. Children. 11(5):566.
- DeLaGarza-Pineda O, Chang T. (2020). Amplitude-Integrated EEG in the Neonatal Intensive Care Unit. In: Sansevere AJ, Harrar DB, eds. Atlas of Pediatric and Neonatal ICU EEG. 1st ed. Springer Publishing Company.
- Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. (2011). The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 28(6):611-617.
- Hellström-Westas L, Rosén I, De Vries LS, Greisen G. (2006). Amplitude-integrated EEG Classification and Interpretation in Preterm and Term Infants. NeoReviews. 7(2):e76-e87.
- Durrani NUR, Dinan MH. (2022). Amplitude-Integrated Electroencephalography: A Primer for Neonatologists and Practitioners in the NICU. NeoReviews. 23(2):e96-e107.
- Castro JSD, Leslie ATFS, Guinsburg R. (2020). Perinatal factors associated with amplitude-integrated electroencephalography abnormalities in preterm infants on the first day of life. J Pediatr (Rio J). 96(5):644-651.
- Boylan GB, Kharoshankaya L, Mathieson SR. (2019). Diagnosis of seizures and encephalopathy using conventional EEG and amplitude integrated EEG. In: Handbook of Clinical Neurology. Vol 162. Elsevier. pp. 363-400.
- Hellström-Westas L. (2018). Amplitude-integrated electroencephalography for seizure detection in newborn infants. Semin Fetal Neonatal Med. 23(3):175-182.
- Rana D, Pollard L, Rowland J, Dhanireddy R, Pourcyrous M. (2020). Amplitude-integrated EEG in infants with neonatal abstinence syndrome. J Neonatal-Perinat Med. 12(4):391-397.
- Obeid R, Tsuchida TN. (2016). Treatment Effects on Neonatal EEG. J Clin Neurophysiol. 33(5):376-381.
- Pressler RM, Abend NS, Auvin S, Boylan G, Brigo F, Cilio MR, et al. (2023). Treatment of seizures in the neonate: Guidelines and consensus?based recommendations—Special report from the ILAE Task Force on Neonatal Seizures. Epilepsia. 64(10):2550-2570.
- Thoresen M, Hellström-Westas L, Liu X, De Vries LS. (2010). Effect of Hypothermia on Amplitude-Integrated Electroencephalogram in Infants With Asphyxia. Pediatrics. 126(1):e131-e139.
- Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. (2017). Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 318(1):57.
- Karpi?ski ?, Mazela J. (2015). Amplitude-Integrated Electroencephalography Use in Preterm Infants: Current Knowledge and Applications. NeoReviews. 16(9):e526-e534.
- Shah DK, Zempel J, Barton T, Lukas K, Inder TE. (2010). Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 67(1):102-106.
- Chandrasekaran M, Chaban B, Montaldo P, Thayyil S. (2017). Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J Perinatol. 37(6):684-689.
- Wang X, Borovac A, Van Den Hoogen A, Tataranno ML, Benders MJNL, Dudink J. (2024). Nurses’ experiences and perspectives on aEEG monitoring in neonatal care: A qualitative study. J Neonatal Nurs. 30(2):165-170.
- O’Sullivan M, Temko A, Bocchino A, O’Mahony C, Boylan G, Popovici E. (2019). Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors. 19(11):2637.
- Tsoi K, Yam KKM, Cheung HM, Ma TPY, So KW, Fung ELW, et al. (2024). Improving Consistency and Accuracy of Neonatal Amplitude-Integrated Electroencephalography. Am J Perinatol. 41(03):330-336.
- Fabregat-Sanjuan A, Rodríguez-Ballabriga Á, Rigo-Vidal A, Pàmies-Vilà R, Larrosa-Capaces S, Rius-Costa V, et al. (2024). Analysis of electrode performance on amplitude integrated electroencephalography in neonates: evaluation of a new electrode aCUP-E vs. liquid gel electrodes. Front Pediatr. 12:1452862.
 Abstract
Abstract  PDF
PDF