Past Issues
Use of Laryngeal Mask Airway as a Bridge to Extracorporeal Membrane Oxygenation in a Neonate with Undiagnosed Tracheal Stenosis
Lauren E. Swanson1*, Jamie Gilley1, Prakash Masand2,Shar ada Gowda1
1Department of Pediatrics, Division of Neonatology at Texas Children’s Hospital; Baylor College of Medicine; Houston, TX 2Department of Pediatrics, Division of Pediatric Radiology at Texas Children’s Hospital; Baylor College of Medicine; Houston, TX
*Corresponding author: Lauren E. Swanson, Department of Pediatrics, Division of Neonatology at Texas Children’s Hospital; Baylor College of Medicine; Houston, TX 77030, USA, Tel: (412)-952-6445; Fax: (832)-825-1386; E-mail: [email protected]. Received: July 07, 2020 Publication: October 22, 2020
Citation: Swanson LE. (2020). Use of Laryngeal Mask Airway as a Bridge to Extracorporeal Membrane Oxygenation in a Neonate with Undiagnosed Tracheal Stenosis. Neonatal. 1(1):01.
Citation: Swanson LE. (2020).
ABSTRACT
We describe a 37-week gestation infant admitted to the neonatal intensive care unit with prenatal diagnosis of Trisomy 21 and atrioventricular canal. Initial course was uneventful. He required high-flow nasal cannula and diuretics secondary to pulmonary over-circulation. Due to inadequate oral feeding despite optimizing therapies, the infant underwent gastrostomy tube surgery. He was intubated in the operating room by anesthesia. Following extubation, he developed respiratory distress unresponsive to racemic epinephrine and dexamethasone. He was unable to be reintubated beyond the level of the vocal folds despite attempts by neonatology, anesthesia, and otolaryngology. A laryngeal mask airway was inserted. The patient was transported for computed tomography of the airway to establish the anatomy and site of obstruction. Imaging revealed long-segment tracheal stenosis with complete cartilaginous rings. In the setting of both airway obstruction requiring tracheoplasty as well as atrioventricular canal defect requiring repair, the multidisciplinary team decided to cannulate the infant for veno-arterial extracorporeal membrane oxygenation. This is the first known neonatal case involving use of laryngeal mask airway as a bridge first to diagnostic imaging then to extracorporeal membrane oxygenation in a patient with critical airway malformation and congenital heart disease within the first year of life. We encourage readers to think of congenital tracheal stenosis as a rare differential diagnosis in the evaluation of an infant with post-extubation respiratory distress of unclear etiology. We also highlight the viability of laryngeal mask airway as a bridge to diagnostic imaging before cannulation for extracorporeal life support.
KEYWORDS: Airway Malformation; Laryngeal Mask Airway; Congenital Tracheal Stenosis; Extracorporeal Membrane Oxygenation
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) has been increasingly used over recent years to provide cardio-pulmonary support to neonates with cardio-pulmonary failure [1]. This report highlights the first known pediatric case involving use of laryngeal mask airway (LMA) as a bridge to computed tomography (CT) for diagnosis of critical airway malformation and then to ECMO in a patient diagnosed with congenital tracheal stenosis, congenital heart disease, and trisomy 21. The normal trachea consists of C-shaped rings of cartilage which gives the trachea its unique anatomical and physiological stability [2]. In the case of congenital tracheal stenosis, the tracheal diameter is narrowed by more than 50% and often fixed by O-shaped cartilaginous rings which do not allow for growth [2]. Treatment in severe cases of long segment tracheal stenosis includes slide tracheoplasty [3]. ECMO may be considered in patients with severe stenosis if they are unable to tolerate conventional methods of ventilation [3].
CASE
Here we describe a 37-week gestation African American male infant admitted to the neonatal intensive care unit (NICU) with prenatal suspicion for Trisomy 21 and balanced atrioventricular (AV) canal. A postnatal echocardiogram (ECHO) revealed a common AV valve, large primum atrial septal defect (ASD), large ventricular septal defect (VSD) with common AV valve regurgitation, and aortic valve with partial fusion of the right and non-coronary cusps. Postnatal genetic testing confirmed Trisomy 21. At birth, the infant initially had low oxygen saturation by pulse oximetry requiring blow-by oxygen and continuous positive airway pressure (CPAP) for approximately 15 minutes. Apgar scores were 7 and 8 at 1 minute and 5 minutes of life, respectively. He was transferred to the NICU in room air. He initially remained stable in room air with gradually increasing tachypnea over the next few weeks. On day of life 35, respiratory support was initiated with high-flow nasal cannula (HFNC) 2 liters per minute (L/min) with fraction of inspired oxygen (FiO2) of 21%. At this point, it should be mentioned that several notes early in his hospital course documentnasal congestion and noisy breath sounds in the absence of labored breathing; however, the primary etiology of his respiratory insufficiency was thought to be secondary to pulmonary over-circulation in the setting of left-to-right shunting through the AV septal defect. On day of life 42, he was initiated on furosemide. The infant remained stable on HFNC 2 L/min, FiO2 21%, with improving tachypnea on diuretic therapy, until day of life 59, at which time he underwent gastrostomy tube (GT) surgery due to inadequate oral feeding. In the operating room, the infant was intubated for the first time in his life by anesthesia with a 3.0 cuffed endo-tracheal tube (ETT).
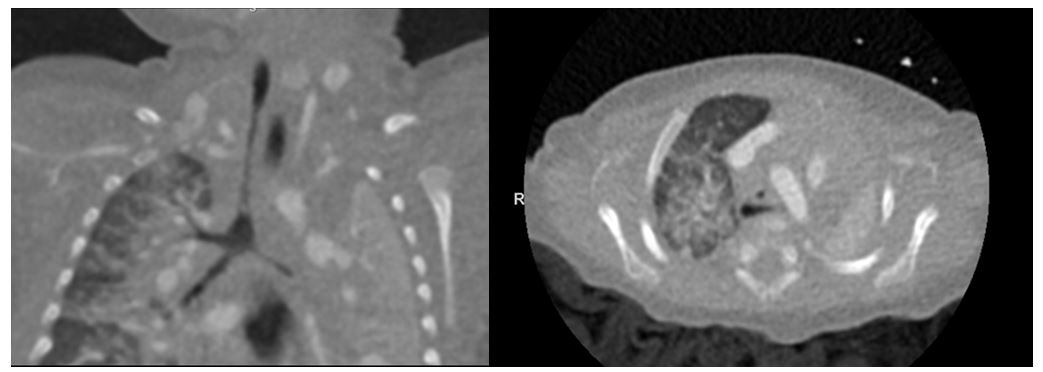
Upon readmission to the NICU post-GT placement, a chest radiograph confirmed the ETT was positioned high in the trachea above the thoracic inlet. He was on minimal ventilator settings hence extubated post-operatively after anesthesia wore off. He then developed significant respiratory distress characterized by stridor, retractions, and hypoxia that was unresponsive to racemic epinephrine and dexamethasone. Reintubation was attempted by the neonatology service with a 3.0 ETT with agrade I view of the vocal folds. The ETT was unable to pass through the vocal folds. The otolaryngology (ENT) service was paged urgently and performed a rigid bedside bronchoscopy which revealed severe tracheal stenosis, unable to rule out complete tracheal rings. After unsuccessful attempts by both ENT and anesthesia teams to reintubate in the NICU, the decision was made to place a laryngeal mask airway (LMA) and transport to radiology for further description of the anatomic obstruction. Dynamic CT of the airway revealed the diagnosis of long-segment tracheal stenosis (over 2.7 cm in length with minimum tracheal diameter 1.5mm) extending from the level of the thoracic inlet to just above the carina (Figure 1). The persistent round configuration of the stenotic segment was suggestive of underlying complete cartilaginous rings.
Figure 1: Long-segment Tracheal stenosis on Dynamic Airway Computed Tomographyin Coronal View (left) and Axial View (right). Severe tracheal narrowing seen extending from thoracic inlet to just above the carina. Stenotic segment length >2.7cm with minimum tracheal dimension measuring 1.5mm. Involvement of the proximal left main stem bronchus over length of 7mm. Left lung collapse with compensatory mild hyperinflation of right lung.
Multiple efforts to maximize ventilation and oxygenation were attempted after the diagnosis of long-segment tracheal stenosis was confirmed. The infant continued to have increased work of breathing with accompanied respiratory failure evidenced by worsening ventilation and oxygenation. Conventional mechanical ventilation with maximal settings was utilized without much improvement. Once the diagnosis was established, a multidisciplinary team met to establish goals of care. The team decided to cannulate the infant for ECMO due to impending respiratory failure. In the context of concurrent congenital heart disease requiring surgical repair, decision was made to cannulate for VA-ECMO. Ventilator settings on the LMA were PIP 38, PEEP 9, rate 50, and 100% FiO2. Arterial blood gas prior to ECMO cannulation revealed pH 7.15, PCO2 73, PaO235, bicarbonate 27, and base deficit -9 with lactate 0.9. Oxygenation index at this point in time with respiratory support was 40.
VA-ECMO was initiated in preparation for slide tracheoplasty and cardiac repair with airway and cardiothoracic surgery team collaboration. He underwent slide tracheoplasty by ENT and pulmonary artery (PA) banding by cardiothoracic surgery on day 4 of VA-ECMO. This case features the safe and successful use of laryngeal mask airway during diagnostic CT imaging followed by ECMO cannulation in anticipation of slide tracheoplasty and PA banding.
DISCUSSION
Congenital tracheal stenosis is extremely rare with an estimated incidence of 1 in 64,500 live births [4]. Each case is managed differently depending on the anatomic location and extent of stenosis, involvement of the trachea and bronchi, and presence of complete tracheal rings [4]. Options described for maintaining oxygenation and ventilation in these difficult cases are varied; these include stenting open the stenotic segment, intubation of the distal trachea, single-lung ventilation via selective intubation of a bronchus, supraglottic jet, and cardiopulmonary bypass [5]. Ventilation strategy in adult patients with severe tracheal stenosis undergoing urgent tracheal stenting can consist of utilizing an LMA if the stenotic area encompasses the upper third of the trachea [5]. Additionally, a retrospective analysis of open tracheal surgery for tracheal stenosis in adults considered LMA’s as a feasible airway alternative for airway management and ventilation in adults [6]. The usage of LMA as a safe airway alternative in adults undergoing tracheal stenosis surgery has been well described in adult literature; however, there are far fewer publications on its usage and safety in neonates with congenital tracheal stenosis [6-10]. The authors postulate that this disparity may be due to the rarity of the diagnosis as well as the frequent necessity for open repair in many neonates with congenital tracheal stenosis due to the technical challenge of passing surgical instruments through a small LMA. In adults, an endoluminal approach has been commonly used due to the larger LMA size preventing obstruction of ventilation when tools are inserted [8]. With so few pediatric cases reported, it is imperative to bring awareness to the medical community of options for preserving adequate gas exchange in pediatric patients who have respiratory distress due to congenital airway malformations but cannot be intubated.
Ours is the first known neonatal case involving emergent use of LMA as a bridge to detailed diagnostic imaging for surgical planning followed by cannulation for VA-ECMO, particularly in a patient diagnosed with a critical airway malformation late in life despite a prolonged NICU stay along with unrepaired congenital heart disease and Trisomy 21. There is one reported case in the medical literature of the use of LMA as a bridge to veno-venous ECMO (VV-ECMO) in a 6-month-old infant who had been hospitalized multiple times for respiratory distress with upper respiratory infections who later was found to have congenital tracheal stenosis [11]. Fuzaylov and Cauley described this case in which VV-ECMO was used as a means of maintaining oxygenation and ventilation for two-hour duration during surgery in a patient otherwise unable to be intubated endotracheally [11]. Our presented case is unique in several ways. For our patient, the LMA was used as a rescue airway in an unstable patient prior to the diagnosis of long-segment tracheal stenosis being known. It is our belief that an emergency tracheotomy prior to diagnostic imaging would have been unsuccessful due to this patient’s long stenotic segment and likely would have placed this infant at high risk for morbidity and mortality. The LMA was also a temporizing measure until the infant could be cannulated onto ECMO for a more stable source of oxygenation and ventilation during surgery. Because our infant also had congenital heart disease which would eventually require cardiopulmonary bypass for repair, the surgical team made a timely decision to cannulate onto VA-ECMO, rather than VV-ECMO, in anticipation of future surgeries the infant would require.
Other literature on the topic includes an adult anesthesia case series reporting five out of 22 cases of acquired tracheal stenosis requiring cardiopulmonary bypass due to the location of the stenotic segment, with the remaining cases able to maintain spontaneous ventilation through an alternate airway, including LMA [12]. A pediatric case series by Huang et al. described three cases in which VA-ECMO was established in pediatric patients due to congenital airway malformations precluding endotracheal intubation [12]. The three cases described included a left pulmonary artery sling with long-segment tracheal stenosis, a patient with abnormal origin of the right bronchus arising from the left main bronchus, and one with lung agenesis with long-segment tracheobronchial stenosis [12]. In these cases, VA-ECMO was used successfully in a planned setting peri-operatively during surgical repair of the airways, with the diagnoses known prior to initiation of ECMO. Unlike our case, all three patients were able to come off ECMO fairly quickly postoperatively. Our patient, who previously had not shown any specific symptoms of upper airway obstruction, presented unexpectedly with respiratory distress and stridor following extubation after a gastrostomy tube placement and required an emergent alternate airway. This account shines a light on congenital tracheal stenosis as a rare differential diagnosis in the evaluation of a formerly asymptomatic infant with post-extubation respiratory distress of unclear etiology. Congenital tracheal stenosis often goes undiagnosed due to its rarity and its symptoms often overlapping with several other diagnoses including tracheobronchomalacia, tracheal agenesis/atresia, esophageal atresia with tracheoesophageal fistula, extrinsic compression, vascular rings, prolonged intubation, infectious tracheitis, and more.2 It is also frequently associated with congenital heart disease [3].
It is important for clinicians to recognize the presenting symptoms for congenital tracheal stenosis, particularly in cases that are recurrent, unexplained, or when other more common diagnoses have been excluded. These presenting findings include respiratory distress associated with retractions, stridor, episodic dyspnea, repeated respiratory infections, and/or persistent cough [3]. In some cases, such as ours, the diagnosis is made upon failed attempts at intubation [2]. Sandu and Monnier offer useful advice for differentiating these diagnoses and determining the most appropriate steps in management [2]. Prompt recognition of congenital tracheal stenosis and utilizing an LMA as a rescue therapy when intubation fails is a lifesaving intervention [9].
CONCLUSION
In conclusion, it is imperative for pediatric clinicians to be cognizant of congenital tracheal stenosis as a rare but potentially life-threatening diagnosis in infants with respiratory distress of unclear etiology. Early recognition and evaluation for ECMO candidacy may prevent morbidity and mortality. The use of LMA was a temporarily life-saving intervention for the presented patient in our NICU by allowing our team to provide a stable emergency airway both for detailed diagnostic imaging and as a bridge to ECMO. We believe that having the results of diagnostic imaging revealing a critical long-segment tracheal stenosis saved this patient from a difficult or unsuccessful emergency tracheotomy, which is often the next step in cases of airway malformations refractory to endotracheal intubation. We also emphasize necessity of a highly skilled neonatal and surgical subspecialty team in the coordination of care required for these patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose. No funding was obtained for the purposes of writing or publishing this case report.
REFERENCES
- Fletcher K, Chapman R, Keene S. (2018). An overview of medical ECMO for neonates. SeminPerinatol. 42(2):68-79.
- Sandu K, Monnier P. (2007). Congenital Tracheal Anomalies. Otolaryngol. Clin North Am. 40(1):193-217.
- Hoffer ME, Tom LWC, Wetmore RF, Handler SD, Potsic WP. (1994). Congenital Tracheal Stenosis: The Otolaryngologist's Perspective. Arch Otolaryngol Head Neck Surg. 120(4):449–453.
- Hewitt RJ, Butler CR, Maughan EF, Elliot MJ (2016). Congenital tracheobronchial stenosis. Semin Pediatr Surg. 25(3):144-149.
- Zhu JH, Lei M, Chen EG, Qiao Q, Zhong TD. (2018). Ventilation strategy and anesthesia management in patients with severe tracheal stenosis undergoing urgent tracheal stenting. Acta Anaesthesiol Scand. 62(5):600-607.
- Krecmerova M, Schutzner J, Michalek P, Johnson P, Vymazal T. (2018). Laryngeal mask for airway management in open tracheal surgery-a retrospective analysis of 54 cases. J Thorac Dis. 10(5):2567-2572.
- Biro P, Hegi TR, Weder W, Spahn DR. (2001). Laryngeal mask airway and high-frequency jet ventilation for the resection of a high-grade upper tracheal stenosis. J Clin Anesth. 13:141-3.
- Vorasubin N, Vira D, Jamal N, Chhetri DK. (2014). Airway Management and Endoscopic Treatment of Subglottic and Tracheal Stenosis: The Laryngeal Mask Airway Technique. Ann Oto Rhino Laryng. 123(4):293-298.
- Agarwal A, Nakao M, Rajadurai VS, Chandran S. (2017). Neonatal airway: challenging endotracheal intubation in infants with tracheal malformations at birth. BMJ Case Rep. bcr-2016-218818.
- Nelson J, Lee H, Sinha P, Deutsch N. (2018). Management of complete tracheal rings in a neonate with tetralogy of Fallot. BMJ Case Rep. bcr-2018-225392.
- Fuzaylov G, Cauley B. (2012). Spontaneous ventilation via facemask and laryngeal mask airway as bridge to extracorporeal membrane oxygenation during long-segment tracheal stenosis repair. Pediatric Anesthesia. 22:1224-1226.
- Huang SC, Wu WT, Chi NH, Chiu SN, Huang PM, et al. (2007). Perioperative extracorporeal membrane oxygenation support for critical pediatric airway surgery. Eur J Pediatr. 166(11):1129-33.
Copyright: Swanson LE, et al. ©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Swanson LE. (2020). Use of Laryngeal Mask Airway as a Bridge to Extracorporeal Membrane Oxygenation in a Neonate with Undiagnosed Tracheal Stenosis. Neonatal. 1(1):01.
 Abstract
Abstract  PDF
PDF