Current Issue
Systemic Corticosteroids in Refractory Pulmonary Interstitial Emphysema: A Case Report in a Preterm Infant
Ines Johri1,*, Lissa De Potter2, Elise A Verhagen3
1Department of Paediatrics and Neonatology, Kidz Health Castle Universitair Ziekenhuis Brussel, Brussels, Belgium
2Department of Neonatology, Universitair Ziekenhuis Brussel, Brussels, Belgium
3Department of Pediatrics, subdivision of Neonatology, Curacao Medical Center, Willemstad, Curacao
*Corresponding author: Ines Johri, Department of Paediatrics and Neonatology, Kidz Health Castle Universitair Ziekenhuis Brussel, UZ Brussel Brussels Health Campus Laarbeeklaan 101 1090 Jette, Brussels, Belgium, Phone: +32486084725, E-mails: [email protected]; [email protected]
Received Date: August 15, 2025
Published Date: November 10, 2025
Citation: Johri I, et al. (2025). Systemic Corticosteroids in Refractory Pulmonary Interstitial Emphysema: A Case Report in a Preterm Infant. Neonatal. 6(2):27.
Copyrights: Johri I, et al. © (2025).
ABSTRACT
Background: Pulmonary interstitial emphysema (PIE) is characterized by alveolar and airway overdistension with rupture into the interstitial space, forming cystic air collections. It occurs predominantly in preterm infants with neonatal respiratory distress syndrome (nRDS) requiring mechanical ventilation. Case presentation: We report a male infant born at 27 4/7 weeks, who developed severe bilateral PIE in the first days of life. Conservative management, including lung-protective ventilation and lateral positioning, failed. On day of life (DOL) 11, systemic dexamethasone was initiated (starting dose 0.3 mg/kg/day). Progressive clinical and radiographic improvement was observed, with successful extubation on DOL 15 and sustained weaning on non-invasive support. The total dexamethasone course lasted 22 days, with a cumulative dose of 3.15 mg/kg. The infant was discharged home on DOL 90 with supplemental oxygen, discontinued at 8 months. At the most recent follow-up (22 months corrected age), he shows good growth favorable psychomotor development. Conclusion: This case highlights the potential role of systemic corticosteroids in refractory PIE. By reducing airway inflammation, edema and interstitial air trapping, steroids may facilitate extubation and recovery. However, the optimal regimen and long-term safety remain uncertain. Corticosteroid therapy should be considered in severe PIE unresponsive to conservative measures, while further research is needed to define best practices.
Keywords: Pulmonary Interstitial Emphysema, Pregnancy, Lung Damage, Cytokines
INTRODUCTION
Pulmonary interstitial emphysema (PIE) is a pathology characterised by alveolar and airway overdistension leading to tears, resulting in air leaks in the interstitial space. Through a one-way valve process, cystic spaces are formed. PIE mainly affects premature infants with neonatal respiratory distress syndrome (nRDS) and is associated with pneumothorax, higher mortality and bronchopulmonary dysplasia (BPD) [1,2]. Since routine surfactant administration and lung protective ventilation strategies are standard practices in neonatology, the incidence of PIE has decreased [3,4]. Furthermore, early surfactant administration as opposed to late is associated with less development of PIE [1]. It is known that positive pressure ventilation can lead to overdistension. Therefore, logically, it is an important factor contributing to the development of PIE [2].
CASE PRESENTATION
Perinatal history
We report a male infant born at 27 4/7 weeks with a birthweight of 1300 g. The pregnancy was uncomplicated until preterm labor. Delivery occurred unexpectedly at home. No antenatal corticosteroids were given.
Initial management
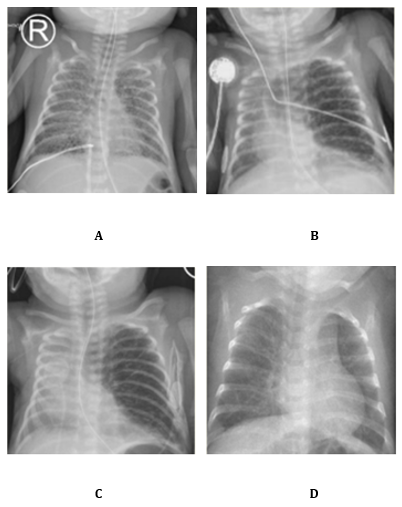
Due to severe nRDS, three doses of surfactant were administered. The first was given at 3 hours of life using less invasive surfactant administration (LISA). At 4 hours, he was intubated and placed on high-frequency oscillation (HFO) ventilation. Ventilation settings included mean airway pressure (MAP) of 8-10 cmH?O and a maximal amplitude of 28. Permissive hypercapnia was accepted with PaCO? up to 65 mmHg. Despite these measures, the respiratory condition deteriorated. Chest radiography revealed severe bilateral PIE (Image 1A). Conservative management was started, consisting of strict lateral positioning and strict lung-protective ventilation strategies. The main challenge was to maintain adequate MAP for gas exchange while avoiding further overdistension.
Clinical deterioration
At one week of life, the infant was extubated to non-invasive neurally adjusted ventilatory assist (NIV-NAVA) with PEEP 8 and level 1 support. Within 24 hours, pulmonary emphysema worsened. Chest X-ray showed marked left lung overexpansion with mediastinal shift to the right (Image 1B). He was re-intubated to prevent further overdistension. Ventilation was continued with HFO alternated with a short period of NAVA. MAP was maintained at 8-10 cmH?O.
Steroid therapy
Because of severe PIE with left lung overexpansion, with selective intubation considered unsafe, high-dose intravenous corticosteroids were initiated by team consensus. On day of life (DOL) 11 treatment started at 0.3 mg/kg/day for three days. Therapy was then switched to the oral route with a gradual taper (Table 1). On DOL 14, the regimen was reduced to 0.2 mg/kg/day in two oral doses (0.13 mg each). The infant was successfully extubated on DOL 15 (Image 1C).
Further tapering followed: 0.15 mg/kg/day on DOL 16, 0.10 mg/kg/day on DOL 18, 0.05 mg/kg/day on DOL 21. However, due to progressive tachydyspnoea and oxygen dependency under CPAP (PEEP 6.5, FiO? 40–55%), the dose was temporarily increased to 0.15 mg/kg/day on DOL 22. The cumulative dose at that point was 1.8 mg/kg. From DOL 26 onward, gradual tapering resumed: 0.12 mg/kg/day, 0.10 mg/kg/day on DOL 27, 0.08 mg/kg/day on DOL 29 and 0.05 mg/kg/day on DOL 31. The final dose was administered on DOL 33 (Image 1C). The total cumulative dose over the 22-day course was 3.15 mg/kg. The rationale for this regimen was to reduce airway inflammation, improve gas exchange and facilitate extubation. The initial high dose was chosen to achieve a rapid effect, followed by a slow taper to allowing stable weaning on non-invasive support, avoiding rebound deterioration and preventing re-intubation.
Outcome
At 31 weeks postnatal age, low-dose CT of the thorax showed a bulla, consistent with persistent PIE. The infant developed severe BPD, confirmed by repeated failed oximetry tests. He was discharged on DOL 90 with home oxygen therapy (0.5 L/min). Oxygen was discontinued at the calendar age of 8 months. Follow-up at 12 months corrected age, showed a good evolution under inhalation corticosteroids and respiratory physiotherapy with moments of exertional dyspnoea. Cerebral MRI was normal (Kidokoro score 1). At the most recent follow-up at 22 months, the infant remains on inhaled corticosteroids with a planned dose reduction. A repeat CT is planned at 2 years of age. Growth has been favorable. Neurodevelopmental assessments are ongoing and have shown age-appropriate psychomotor development. Given the initial severity of the disease, the long-term outcome is better than expected.
Image 1. A Bilateral PIE , B Unilateral PIE, left lung overdistension with mediastinal shift , C Unilateral PIE with diminution of overdistension D No signs of PIE or overdistension.
Table 1. Corticosteroids dosing regimen
|
Day of Life (DOL) |
Dose (mg/kg/day) |
Route |
Cumulative Dose (mg/kg) |
|
11–13 |
0.3 |
IV |
0.9 |
|
14–15 |
0.2 |
PO |
0.9 |
|
16–17 |
0.15 |
PO |
1.15 |
|
18–20 |
0.10 |
PO |
1.45 |
|
21 |
0.05 |
PO |
1.60 |
|
22–25 |
0.15 |
PO |
1.80 |
|
26 |
0.12 |
PO |
2.45 |
|
27 |
0.10 |
PO |
2.57 |
|
29–30 |
0.08 |
PO |
2.77 |
|
31–32 |
0.05 |
PO |
2.93 |
|
33 |
0.05 |
PO |
3.15 |
DISCUSSION
Considering the initial poor prognosis in our case due to advanced lung damage and the remarkably favourable evolution following administration of high-dose dexamethasone, this case report highlights the possible important role of systemic corticosteroids in the resolution of refractory PIE. Literature about management of PIE in neonates is limited and consists mainly of successful case reports. Conservative approaches ranging from decubital positioning and lung-protective ventilation to selective intubation have been described [2,5].
Mahapatra et al. reported a similar case of a preterm infant with life-threatening PIE and mediastinal shift who responded to hydrocortisone [2]. Mohsini et al. described three cases of acquired lobar emphysema treated with dexamethasone. Two infants improved and were discharged, while one died despite radiographic resolution [6]. Ali et al. presented a case requiring multiple steroid courses, where eventual extubation and long-term recovery were achieved [7]. Fitzgerald et al. reviewed ten infants and found consistent improvement after a short 3-day dexamethasone regimen [8]. Taken together, these reports, including our case, support the view that corticosteroids can be effective in selected infants with refractory PIE, although responses vary depending on disease severity, timing, and duration of therapy (Table 2).
The biological plausibility of corticosteroid benefit in PIE is strong. Corticosteroids have several beneficial effects on lung disease by decreasing inflammation. They promote anti-inflammatory cytokines (interleukin-10), decrease lymphocyte counts and suppress pro-inflammatory cytokines (tumor necrosis factor-alpha and interleukin-1β) [9,10]. They reduce peribronchiolar inflammation and airway wall edema, thereby decreasing small airway obstruction and improving expiratory flow [11]. They stabilize the alveolar–capillary barrier and limit further interstitial air leakage [12]. Upregulation of surfactant protein expression has been demonstrated, which may contribute to improved alveolar stability [13]. Corticosteroids also modulate fibroblast activity and collagen deposition, potentially limiting cystic remodeling that can perpetuate air trapping [14]. These combined mechanisms provide a rationale for their observed clinical effect in PIE.
Despite encouraging outcomes, several questions remain unanswered. The optimal corticosteroid type is unknown. Hydrocortisone may be safer with respect to neurodevelopment but may provide less potent anti-inflammatory activity than dexamethasone [15,16]. The ideal dose and cumulative exposure are also undefined. Short regimens of 3–7 days have been effective in some reports mentioned earlier, while our patient required a 22-day course. This raises concern about prolonged exposure but also suggests that severe cases may require extended therapy. Timing is another unresolved issue. It is unclear whether early initiation in infants with evolving PIE could prevent progression or whether corticosteroids are most useful as a late rescue therapy.
It is well known that corticosteroids are associated with adverse effects, including impaired neurocognitive outcomes and adrenal suppression with adrenal insufficiency [17,18]. Importantly, according to the recent Cochrane review by Onland et al, the effects of postnatal corticosteroid treatment on long-term neurodevelopmental outcomes remain uncertain, underscoring the need for cautious and individualized use in preterm infants [19]. Furthermore, a recent meta-analysis demonstrated that postnatal corticosteroids in newborn animals leads to persistent alveolar simplification [20]. These risks must be balanced against the potentially fatal consequences of uncontrolled PIE. Individualized decision-making, weighing risks and benefits in multidisciplinary discussions, is essential.
Our report also highlights the challenge of clinical decision-making in severe PIE. Selective mainstem intubation is often considered but carries significant risks. It could lead to atelectasis, contralateral hyperinflation, mucosal injury, and hemodynamic instability [1]. In our patient, this approach was avoided and the favorable outcome was likely facilitated by corticosteroid therapy.
|
Study |
Condition |
Steroid & regimen |
Start timing (DOL) |
Duration |
Response / time to response |
Extubation |
Final outcome |
|
Mahapatra et al. |
Refractory pulmonary interstitial emphysema (PIE) |
Hydrocortisone: 2 mg/kg IV once; then 1 mg/kg IV q12h × 48 h |
18 |
≈48 h |
Extubated to nasal CPAP by DOL 19; complete clinical recovery |
DOL 19 |
Long?term: neurodevelopmentally normal; good pulmonary function |
|
Mohsini et al. – Pt 1 |
Acquired lobar emphysema |
Dexamethasone: 0.5 mg/kg/day |
NR |
7 days |
Radiologic resolution within 72 h; improved VR (MVI), AaDO2, PaCO2 |
NR |
Discharged at 10 wks of age |
|
Mohsini et al. – Pt 2 |
Acquired lobar emphysema |
Dexamethasone: 0.5 mg/kg/day, 5 days then tapered over 1 week |
NR |
≈12 days incl. taper |
Dramatic radiologic & gas exchange improvement, but remained ventilator?dependent |
No |
Died at 5 months (cor pulmonale) |
|
Mohsini et al. – Pt 3 |
Acquired lobar emphysema |
Dexamethasone: same dosage as Pt 2 (0.5 mg/kg/day ×5 days then taper 1 week) |
NR |
≈12 days incl. taper |
Complete radiologic resolution during therapy |
During therapy |
Discharged at 9 wks of age; symptom?free |
|
Ali et al. |
Refractory PIE in extreme premature newborn |
Day 20: DART dexamethasone (10?day course) – unsuccessful; Day 31: Hydrocortisone ×3 days; Day 47: DART restarted |
20, 31, 47 |
Multiple courses (10 d; 3 d; 10 d) |
Extubated DOL 48; no recurrence of overexpansion despite reintubations & viral infection |
DOL 48 |
Discharged DOL 134 on room air; thriving on follow?up |
|
Fitzgerald et al. (retrospective case review, n=10) |
PIE in preterm infants
|
Dexamethasone: 0.5 mg/kg/day
|
NR
|
3 days |
Significant clinical improvement in most infants |
NR |
9/10 survived to term |
Table 1 Published reports of corticosteroid therapy for pulmonary interstitial emphysema (PIE) or acquired lobar emphysema in neonates. DOL = day of life; CPAP = continuous positive airway pressure; VR (MVI) = ventilatory requirement (mean ventilatory index); AaDO? = alveolar–arterial oxygen difference; DART = Dexamethasone: A Randomized Trial protocol; NR = not reported
This case report has inherent limitations. It describes a single patient, which limits generalizability. Selection bias is likely, since successful cases are more often published, while unsuccessful attempts may remain unreported. Although follow-up to 22 months corrected age is reassuring, longer-term outcomes, particularly neurodevelopmental and pulmonary sequelae, remain unknown. In addition, corticosteroid dosing regimens are not standardized across published reports, making it difficult to compare outcomes or to recommend specific protocols. Further multicenter studies or registry data are required to determine optimal practice.
CONCLUSION
Systemic corticosteroids may offer an important therapeutic option in severe, refractory PIE. In this case, dexamethasone enabled extubation and supported successful weaning after failure of conventional measures. Mechanistic plausibility supports their role in reducing inflammation, stabilizing alveoli and limiting further air leak. However, evidence is restricted to case reports and major uncertainties persist regarding optimal drug, dose, duration and timing. Safety concerns, particularly regarding long-term neurodevelopment, remain significant. Corticosteroid therapy should therefore be considered cautiously, as a rescue measure in selected infants, within a multidisciplinary framework. Larger studies are needed to provide clear guidance and to establish the balance between benefit and risk in this vulnerable population.
INFORMED CONSENT
Written informed consent from the parents of the patient is obtained for publication of this case report.
REFERENCES
- Jalota Sahota R, Anjum F. (2025). Pulmonary interstitial emphysema. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Mahapatra S, Scottoline B. (2016). Steroid-induced resolution of refractory pulmonary interstitial emphysema. J Matern Fetal Neonatal Med. 29(24):4092-4095.
- Keszler M, Donn SM, Bucciarelli RL, Alverson DC, Hart M, Lunyong V, et al. (1990). Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. J Pediatr. 116(6):1071-1079.
- Hart SM, McNair M, Gamsu HR, Price JF. (1983). Pulmonary interstitial emphysema in very low birthweight infants. Arch Dis Child. 58(12):932-936.
- Gürakan B, Tarcan A, Arda IS, Co?kun M. (2002). Persistent pulmonary interstitial emphysema in an unventilated neonate. Pediatr Pulmonol. 34(5):409-411.
- Mohsini K, Reid D, Tanswell K. (1987). Resolution of acquired lobar emphysema with dexamethasone therapy. J Pediatr. 111(6):901-904.
- Ali M, Mallett L, Miller G. (2021). Refractory pulmonary interstitial emphysema in an extremely premature newborn. Am J Perinatol Rep. 11:e61-e64.
- Fitzgerald D, Willis D, Usher R, Outerbridge E, Davis GM. (1998). Dexamethasone for pulmonary interstitial emphysema in preterm infants. Biol Neonate. 73(1):34-39.
- Williams DM. (2018). Clinical pharmacology of corticosteroids. Respir Care. 63(6):655-670.
- Gupta S, Donn SM, Bhutani VK. (2011). Postnatal corticosteroids for prevention and treatment of chronic lung disease. Neonatology. 99(2):113-119.
- Bancalari E, Gerhardt T. (1998). Corticosteroids and neonatal chronic lung disease. Semin Neonatol. 3(4):303-311.
- Hamacher J, Hadizamani Y, Borgmann M, Geiser T, Mohaupt M, et al. (2018). Cytokine–ion channel interactions in pulmonary inflammation. Front Immunol. 9:849.
- Lu S, He Y, Liu H, Yang Z, Wang J, Wang S, et al. (2025). Cortisol regulates neonatal lung development via Smoothened. Respir Res. 26(1):16.
- Dik WA, et al. (2002). Lung disease of the preterm infant. Rotterdam: Erasmus MC.
- Melan N, Pradat P, Godbert I, Pastor-Diez B, Basson E, Picaud JC. (2024). Neurodevelopment at 24 months corrected age in extremely preterm infants treated with dexamethasone alternatives during the late postnatal period: a cohort study. Eur J Pediatr. 183(2):677-687.
- Boscarino G, Cardilli V, Conti MG, Liguori F, Repole P, Parisi P, et al. (2024). Outcomes of postnatal systemic corticosteroids administration in ventilated preterm newborns: a systematic review of randomized controlled trials. Front Pediatr. 12:1344337.
- Jobe AH. (2009). Postnatal corticosteroids for bronchopulmonary dysplasia. Clin Perinatol. 36(1):177-188.
- Htun ZT, Schulz EV, Desai RK, Marasch JL, McPherson CC, Mastrandrea LD, et al. (2021). Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol. 41(8):1783-1796.
- Onland W, Offringa M, Cools F, De Jaegere AP, Rademaker K, Blom H, et al. (2011). Systemic Hydrocortisone To Prevent Bronchopulmonary Dysplasia in preterm infants (the SToP-BPD study); a multicenter randomized placebo controlled trial. BMC Pediatr. 11:102.
- Lok IMB, Wever KE, Vliegenthart RJS, Onland W, van Kaam AH, van Tuyl M. (2024). Effects of postnatal corticosteroids on lung development in newborn animals: a systematic review. Pediatr Res. 96(5):1141-1152.
 Abstract
Abstract  PDF
PDF