Past Issues
Quality Improvement in the Management of Neonatal Persistent Pulmonary Hypertension of the Newborn (PPHN) that Reduced the Need for ECMO
Vikranth Bapu Anna Venugopalan*, Shanmugasundaram Sivakumar
Consultant Neonatologists with expertise in Paediatric Cardiology, Birmingham City Hospital, Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, UK
*Corresponding Author: Vikranth Bapu Anna Venugopalan, Consultant Neonatologists with expertise in Paediatric Cardiology, Birmingham City Hospital, Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, UK; Email: [email protected]
Received Date: January 31, 2024
Publication Date: February 28, 2024
Citation: Vikranth Bapu AV, Sivakumar S. (2024). Quality Improvement in the Management of Neonatal Persistent Pulmonary Hypertension of the Newborn (PPHN) that Reduced the Need for ECMO. Neonatal. 4(1):13.
Copyright: Vikranth Bapu AV, et al. © (2024).
ABSTRACT
Aim: To improve the management of neonatal PPHN to reduce the need for ECMO. Methods: Data of babies from 2009 to 2019 who were managed for Neonatal PPHN were analysed. Data from 2009 to 2014 were initially analysed to give an insight into the management of Neonatal PPHN which led to the understanding of various parameters in management which needed to be improved. This led to the active Quality improvement programme with education of doctors and nurses regarding aggressive management of babies with PPHN and this led to successful reduction of babies needing ECMO. Results: We analysed two epochs (Aug 2009- Jan 2014 and Feb 2014 to Dec 2019). Over a five year period (2009-2014), 17 infants developed severe persistent pulmonary hypertension of the newborn (PPHN) and required extra corporeal membrane oxygenation (ECMO) from our neonatal unit. We have approximately 6000 deliveries every year in our maternity unit. There were 3 babies every year (from 2009 till 2014) who were referred for ECMO. The problem was identified when an audit was performed in 2013 regarding management of babies with PPHN and this led to the quality improvement project. It was felt that an earlier diagnosis of PPHN, with prompt escalation of treatment, as per the PPHN guideline; early intubation and optimising ventilation, adequate oxygenation, early initiation of nitric oxide, early use of inotropes and escalation of inotropic support and close control of metabolic parameters would have prevented some of these infants from deteriorating. We implemented an active educational programme for the junior doctors and nurses combined with changes to the blood gas chart; the oxygenation index (OI) calculation printed clearly on the chart, prompting calculation of OI in these babies. We also did a simulation session for the medical and nursing team to emphasize the management of PPHN. These were actively discussed in the Quality improvement meetings and once the plan was in place, there were education sessions with each new intake of doctors, comprising a session with the middle grade doctors (registrars) and senior house officer teaching programme and opportunistic reinforcement of this message during multidisciplinary discussion of babies who were subsequently managed for PPHN. This led to significant reduction of babies needing ECMO to 2 babies in 5 years (compared to 17 in the initial 5 years). This is under active surveillance and continues to have less number of babies needing ECMO. Conclusion: With the active involvement of the medical and the nursing team with proactive education regarding the management of PPHN; the referral rates have been successfully decreased from 3/year (2009 to Jan 2014) to one every 18 to 24 months (2014 to 2023). This has led to significant quality improvement in the care of neonates with PPHN and also helped with saving money for the Trust for such expensive, yet effective treatments.
BACKGROUND
Neonatal respiratory failure occurs in 2/1000 live births and Persistent pulmonary hypertension of the newborn (PPHN) complicates the course of approximately 10% of infants with respiratory failure and is a source of considerable mortality and morbidity.
In simple terms, Persistent pulmonary hypertension of the newborn (PPHN) occurs in neonates if they have not adapted to the extra uterine world. To understand this pathophysiology, it is useful to know the fetal circulation.
Adaptation in the Neonatal Period from Fetal Circulation
Once the baby is born, the lungs (not the placenta) has to serve as an organ for gas exchange and thus a dramatic cardiopulmonary transition occurs at birth with a rapid fall in Pulmonary vascular resistance with a 10 fold rise in pulmonary blood flow. The most critical signals are mechanical distension of the lung, a decrease in Carbon dioxide tension and an increase in oxygen tension in the lungs. This leads to the fall in pulmonary vascular resistance. As this happens, there is a change in the circulation whereby there is increased blood flow to lungs with a preferential blood flow from the right atrium to the right ventricle into the pulmonary artery, functional closure of the ductus arteriosus happens due to higher oxygen tension with a flow from left to right due to higher pressure in the left atrium (due to fall in PVR) and this eventually causes a left to right shunt across the foramen ovale.
There are various reasons why PPHN may occur (see table 1), but prompt and aggressive treatment can often prevent a downward spiral of hypoxia, acidosis and hypotension.
Table 1: Causes of PPHN.
|
Poor adaptation to extra uterine life Lung parenchymal disorders Meconium aspiration syndrome Respiratory distress syndrome Pneumonia/Sepsis Hypoxic ischaemic encephalopathy Congenital malformations of the lung Congenital diaphragmatic hernia Congenital Pulmonary Adenomatoid malformations (CPAM) Congenital lobar Emphysema Idiopathic (very rare in neonates) Duct dependent Congenital heart disease TAPVD (obstructed) In the absence of Respiratory or cardiac causes Arteriovenous (AV) malformations Vein of Galen malformation Hepatic AV fistula Sacrococcygeal teratoma |
Severe PPHN has been estimated to occur in 2 per 1000 of live born term infants (8), and some degree of pulmonary hypertension complicates the course of more than 10% of all neonates with respiratory failure. Because a patent foramen ovale and ductus arteriosus are normally present early in life, elevated pulmonary vascular resistance in the newborn will produce extrapulmonary shunting of blood, leading to severe and potentially unresponsive hypoxemia. Even with appropriate therapy, the mortality for PPHN remains between 5-10%. In addition, approximately 25% of infants with moderate or severe PPHN will exhibit significant neurodevelopmental impairment at 12-24 months (9-11). This important observation may indicate that the underlying disease and/or early therapeutic approaches are associated with early neurological injury.
Diagnosis of PPHN
Clinical examination details will show the following features
- Differential cyanosis (Pre > Post and difference of ≥ 5 %)
- Presents usually within the first few hours
- Respiratory distress
- Murmur of Tricuspid regurgitation (Pansystolic murmur usually in the right lower sternal edge)
- Loud P2 and can have a palpable P2 (felt with the thumb kept in the left upper sternal edge)
Overview of PPHN Management
In neonates, PPHN is usually secondary to an underlying pathology (see causes of PPHN in Table 1) and the most common reason is respiratory cause. Management of PPHN comprises of management of primary pathology with early ventilatory support, optimising ventilation, administration of surfactant if high FiO2 with evidence of meconium aspiration, sepsis (esp GBS), early initiation of Blood pressure support with fluid bolus and inotropes and rapid escalation of the inotropes to achieve higher mean blood pressures. In a ventilated baby, it is prudent to use appropriate sedation with or without paralysing agents to optimize ventilation.
In neonatal PPHN, it is usually an oxygenation problem and maintaining normal saturations of ≥95% is necessary. Apart from the need for intubation and ventilation for any other reason, even if the blood gas exchange is within normal limits, if the baby is needing >40% oxygen, we felt it is better to intubate and ventilate the baby and not delay the procedure. This would need to be followed by insertion of central lines (in early presentation; access via umbilical vessels is possible and this can be used to assess invasive blood pressure and also the need for any inotropes if necessary).
Once babies are ventilated, it is important to maintain adequate gas exchange and to manage metabolic parameters and also need to ensure acidosis is corrected. Surfactant administration has shown to have benefit in babies with PPHN due to secondary surfactant deficiency/activity (in conditions such as Meconium aspiration, sepsis, Pneumonia). It is important to analyze the Oxygenation index (OI) rigorously and if OI is rising to >20, it is vital to start Nitric oxide (Selective Pulmonary Vasodilator) to improve oxygenation and also if the baby is consistently needing 100% oxygen with saturations<95%. OI is a product of Mean airway pressure ((MAP) which can be obtained from the Ventilator) and Fractional Inspired Oxygen (FiO2) divided by Partial pressure of Arterial Oxygen (PaO2) in mm mercury (mm Hg). If the gas machine analysis of PaO2 is in kilo pascals (kPa), this would need to be multiplied by 7.5 to give the measurement in mm Hg.
OI = MAP x FiO2
PaO2 (kPa) x 7.5
It is vital to drive the systemic blood pressure above pulmonary pressures (usually high and may even be supra systemic if severe PPHN. In a term neonate, expected mean blood pressure is ≥40 mmHg and with PPHN, it would be prudent to aim for higher mean blood pressure of >45 to 50 mmHg with the use of inotropes such as Adrenaline, Noradrenaline, Hydrocortisone and Dobutamine. It is important to escalate the inotrope every 20-30 minutes to achieve the expected blood pressures. Echocardiogram can give an estimation of pulmonary blood pressures in the presence of Tricuspid regurgitation.
Echocardiographic Assessment of PPHN
Detailed cardiac assessment includes detailed clinical assessment including an echocardiogram to make a diagnosis of PPHN. It is important to perform a sequential cardiac assessment to assess the structure and connections of the heart and it is important to rule out duct dependent cyanotic congenital heart disease. In simple terms, duct dependent CCHD could be due to right sided obstructive lesions (Tricuspid atresia, Critical pulmonary stenosis or pulmonary atresia) or left sided obstructive lesions (Hypoplastic left heart syndrome or Interrupted aortic arch or severe aortic stenosis or coarctation of aorta). Mixing type of lesions are due to Transposition of the great arteries and Total anomalous pulmonary venous drainage (TAPVD). As highlighted below, if there is right sided dilatation with pure right to left shunt at the atrial level, always be cautious and beware of TAPVD.
In PPHN, Right atrium (RA) and Right ventricle (RV) will be dilated, presence of tricuspid regurgitation (TR), pulmonary regurgitation (PR), shunt across the Patent foramen ovale (PFO) depending on the severity of PPHN (Là R shunt in mild PPHN, bidirectional in moderate to severe) and beware of TAPVD if pure right to left shunt and dilated right side of the heart. There can also be varying degrees of shunting at the level of the PDA (bidirectional shunt and if there is predominantly right to left shunt and PDA and left to right shunt at PFO level, needs a detailed assessment for possibility of Interrupted aortic arch/ coarctation of aorta).
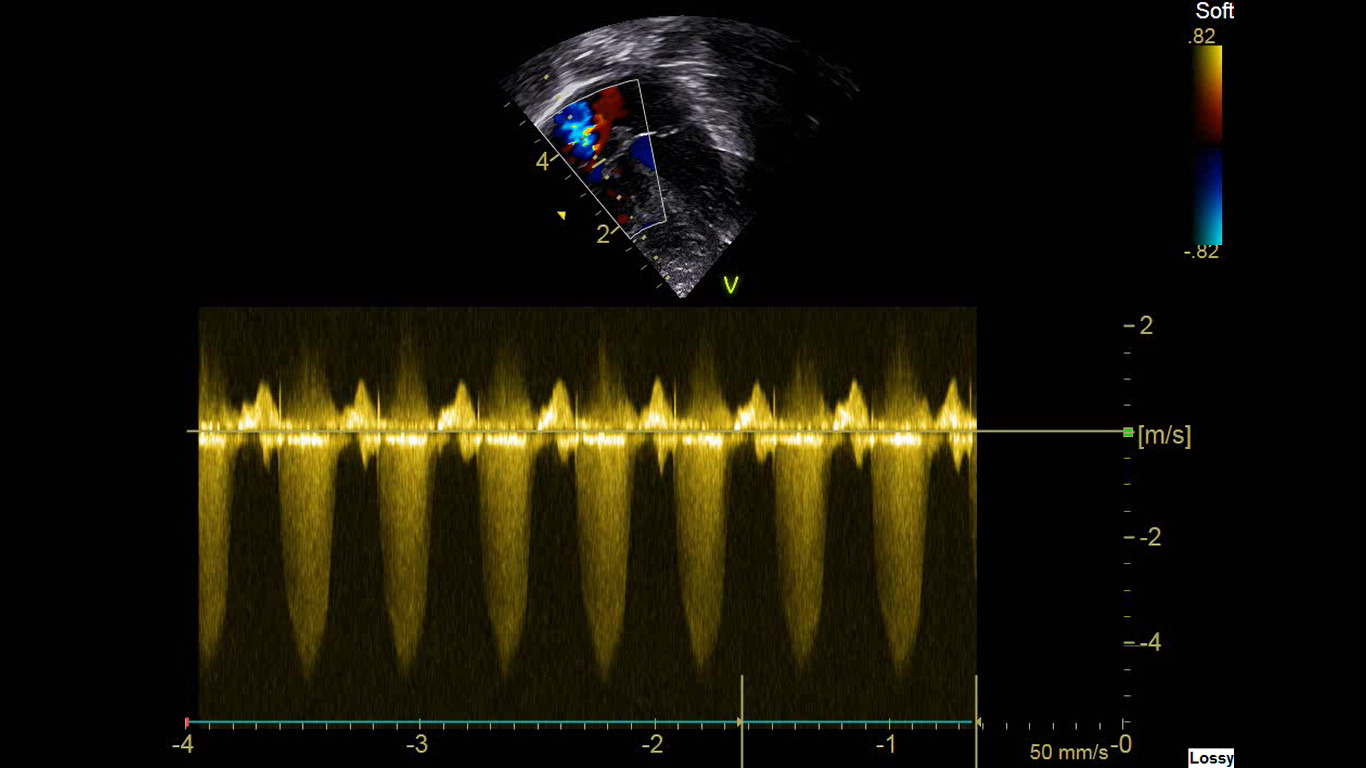
In presence of TR (Fig 1), gradient across the RV and RA can be assessed with modified Bernoulli’s equation
TR Gradient = 4 X (TR velocity Vmax)2
Estimated pulmonary pressures = TR gradient + right atrial pressure (approximately 6-8 mmHg).
Figure 1: Apical 4 chamber view with Tricuspid regurgitation (TR) with a velocity of just >4m/second.
If the velocity (Vmax) is 4 m/second, TR gradient is 4 x (4)2 = 4 x 16 =64mmHg
Estimated PA Pressure = TR gradient + Right Atrial (RA) pressure which is approximately 6-8mm Hg.
Estimated PA pressure is around 70mmHg. In this situation, it is important to keep the systolic systemic blood pressure to be at least above 70mmHg to reduce the right to left shunting. This could be achieved by bolus fluids with colloids and then with inotropes.
PA pressures can also be estimated in presence of PDA or VSD.
Estimated PA pressure = Systolic BP – VSD/PDA gradient (4V2)
If VSD/PDA velocity is 2m/sec and systolic BP is 50mmHg.
Estimated PA pressure = 50-16 = 34 (Just >2/3rd systemic pressures).
Functional assessment of PPHN
LV function
LV Ejection fraction (EF)
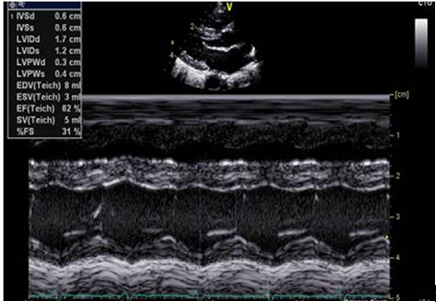
LV Ejection Fraction (Fig 2) is a percentage change of LV volume between end-diastole and end-systole and is measured as a 2D measurement on M mode in PLAX view. Normal range is 55-80% for all ages. (41-55% - mild dysfunction, 31-40% - moderate dysfunction, ≤30% - severe dysfunction)
LV Fractional Shortening (FS%)
LV fractional shortening (Fig 2) is the LV intraluminal diameter change and is measured as a 2D measurement on M mode in PLAX view. Normal range is 28-46%. (20-25% - mild dysfunction, 15-19% - moderate dysfunction, ≤14% - severe dysfunction)
Figure 2: Parasternal long axis view for left ventricular functional assessment showing Ejection fraction (EF) and Fractional shortening (FS)
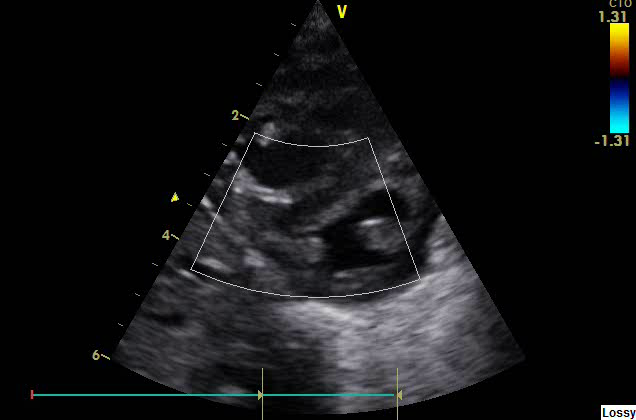
In parasternal short axis view (PSAX), LV will be seen as a circular structure and in PPHN; it can become a D shaped structure (due to RV dilatation and squashing of LV due to high RV pressures).
Figure 3: LV seen as D shaped structure due to PPHN in Parasternal short axis view (PSAX)
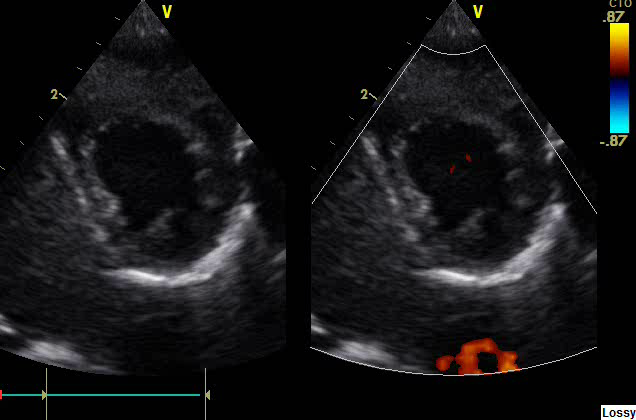
Figure 4: LV seen normally as a circular structure in Parasternal Short axis view (PSAX)
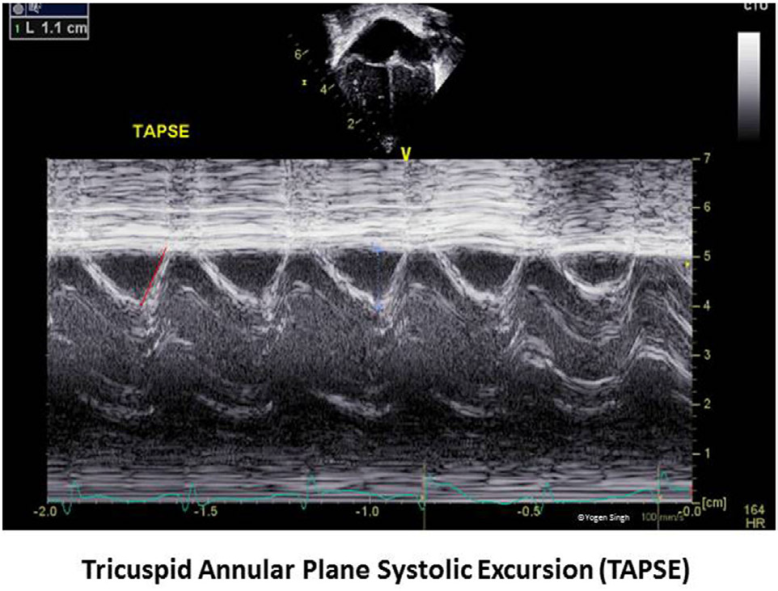
Tricuspid Annular Plane systolic excursion (TAPSE) is the systolic excursion of lateral (or medial) tricuspid annulus toward apex to assess RV function and is assessed in Apical 4 chamber view. In term neonates it is more than 8mm (8-11mm), Z scores available for children and generally >12mm (12-17mm) and in adults or grown-up children it is >17mm (17-25mm).
Figure 5: Tricuspid Annular Phase systolic excursion
LV output (Stroke volume) is the product of VTI measured by pulse wave Doppler at LVOT in Apical 5 chamber view and LVOT cross sectional area measured in PLAX view. Z scores are available for different ages. Neonates: 150-400ml/kg/minute.
RV output (Stroke volume) is the product of VTI measured by pulse wave Doppler at RVOT and RVOT cross sectional area measured in PSAX view. Z scores are available for different ages. Neonates: 150-400ml/kg/minute.
ECG will show evidence of RVH and right axis deviation and tall p waves in V2.
METHODS
We did a retrospective study of babies with PPHN from 2009 to 2014 who required ECMO and this was followed up with ongoing surveillance. We implemented changes such as education of the doctors and nurses to manage the condition effectively and analysed the results of such intervention.
DATA FROM OUR STUDY: In our population with approximately 6000 deliveries every year, we had 37 babies/30000 (1.7/1000) with PPHN between 2009 and 2014. Among these babies, 3 babies needed ECMO every year till Jan 2014 (n=17). None of the babies died, all survived and have been well. In the subsequent years (2014 -2019), the number of babies with PPHN 36 babies/27800 (1.3/1000), but only 2 babies needed ECMO after implementation of the changes which led to significant quality improvement (Table 1).
Table 2: (Years 2009-2019) and the number of babies who needed ECMO. 1 - PPHN Audit done in 2013 identified the issues with the medical management of babies with PPHN. 2 – ECMO Audit done in early 2014 identified the number of babies with PPHN needing ECMO and 3 Institution of Education programme for medical & nursing team as a Quality improvement project
|
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
|
2 |
3 |
3 |
5 |
2 |
2 |
1 |
0 |
1 |
0 |
0 |
Table 3: Correlation between age at admission, age at intubation, surfactant administration, high admission pCO2, ECMO time and Duration in the hospital
NS: Not significant
|
|
Age at admission |
Intubation time |
Admission PaCO2 |
Age surfactant given |
ECMO Time |
Duration in Hospital |
|
Age at admission |
NS |
NS |
NS |
NS |
NS |
NS |
|
Intubation time |
Significant P=0.00008 |
NS |
NS |
NS |
NS |
NS |
|
Admission PaCO2 |
Significant P=0.00546 |
NS |
NS |
NS |
NS |
NS |
|
Age surfactant given |
Significant P=0.03606 |
NS |
Significant P=0.02822 |
NS |
NS |
NS |
|
ECMO time |
NS |
NS |
NS |
NS |
NS |
NS |
|
Duration in hospital |
NS |
NS |
NS |
NS |
NS |
NS |
The above table (Table 2) shows a correlation between age at admission and the timing for intubation and was significantly delayed with many babies managed on non-invasive respiratory support when they were still needing more than 50% Oxygen, surfactant administration was delayed and not given at the time of ventilation and only 70% of the babies received Surfactant. These results suggested that there was a delay in the decision to intubate and ventilate (in spite of high oxygen requirement and higher CO2 levels in the blood gas) and a delay in the administration of surfactant. We also observed that the Oxygenation index was not calculated early enough to initiate measures such as increasing the ventilator parameters and also initiation of Nitric Oxide.
RESULTS
Over a five year period (2009-2014), we had 17 infants who developed severe persistent pulmonary hypertension of the newborn (PPHN), requiring referral to a center for extra corporeal membrane oxygenation (ECMO) from our neonatal unit; catering for approximately 6000 deliveries every year. There were 3 babies every year (from 2009 till 2014) who were referred for ECMO and required ECMO.
The problem was identified when an audit was performed in 2013 (Table 1) regarding management of babies with PPHN; this led to the quality improvement project. It was felt that an earlier diagnosis of PPHN, with prompt escalation of treatment, as per the Neonatal PPHN Network guideline; early intubation and ventilation, oxygenation, early initiation of nitric oxide, early use of inotropes and escalation of inotropic support and close control of metabolic parameters would have prevented some of these infants from deteriorating.
We implemented an active educational programme for the junior doctors and nurses combined with changes to the blood gas chart; the oxygenation index (OI) calculation printed clearly on the chart, prompting calculation of OI in these babies. We also did simulation session for the medical and the nursing team to emphasize the proactive and aggressive management of PPHN. These were actively discussed in the Quality improvement meetings and once the plan was in place, there were education sessions with each new intake of doctors, comprising a session in the registrar and senior house officer teaching programme and opportunistic reinforcement of this message in babies who were subsequently managed for PPHN.
DISCUSSION
The plan, do, study, act (PDSA) cycle was used to structure our quality improvement project. The problem of increased referral for ECMO (three per year) for neonates with PPHN was identified (PLAN) with an audit. There were patients with PPHN whom it was found that their management was still not optimal, with late diagnosis and delayed institution of intervention, as per the PPHN trust guidelines. Therefore the plan was reviewed and it was felt that further reinforcement was needed as new doctors coming through may not appreciate the message as clearly as the original team. Education of the nursing staff was also done, as they are key members of the team who may be the first to identify the first signs of PPHN. The plan to improve care with improved education and the oxygenation index calculation on the blood gas chart was instituted (DO). Once the introduction of these measures, the referral rates for ECMO was reduced to one baby every 18 months to 2 years (STUDY) which deemed the intervention a success! We have actively kept these measures under surveillance (ACT) and continue to have reduced rate of referral for ECMO.
So far the changes made have resulted in better care for our sickest infants with PPHN, with a continued reduced rate of referral for ECMO. The emphasis on continued education and reinforcement for the new medical and nursing staff have been written into the teaching plan and a plan for ongoing surveillance in the management of PPHN to ensure constant reinforcement and discussion about the condition and its management.
This project has reduced our referrals for ECMO, improved the care of infants with PPHN and had a significant financial saving for our hospital trust; but its continued success relies on the continued input and education of our neonatal team and further PDSA cycles.
CONCLUSION
With the continued education of the medical and nursing team regarding early aggressive management of PPHN, the referral rates have been successfully decreased from 3/year (2009 to Jan 2014) to one every 18 months (2014-2015) and then one baby needed ECMO after 2 years in Aug 2017 and none needed ECMO till the end of 2019. This is under surveillance with the ongoing educational program and we have had 4 babies with PPHN who needed ECMO (2020 to 2023) after referring to a tertiary centre. This has led to significant quality improvement in the care of neonates with PPHN and also led to financial savings for the Trust for such expensive, yet effective treatments.
REFERENCES
- UK Collaborative ECMO Trial Group. (1996). UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 348:75-82.
- Bennett CC, Johnson A, Field DJ, Elbourne D. (2001). UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet 357:1094-1096.
- Steinhorn RH. (2010). Neonatal Pulmonary Hypertension. Pediatr Crit Care Med. 11(2 Suppl): S79–S84.
- Field DJ, Firmin R, Azzopardi DV, Cowan F, Juszczak E, Brocklehurst P, et al. (2010). Neonatal ECMO Study of Temperature (NEST) – a randomised controlled trial. BMC Pediatrics. 10:24.
- Tiruvoipati R, Pandya H, Manktelow B, Smith J, Dodkins I, Elbourne D, et al. (2007). Referral pattern of neonates with severe respiratory failure for extracorporeal membrane oxygenation. Arch Dis Child Fetal Neonatal Ed. 93(2):F104-F107.
- Khambekar K, Nichani S, Luyt DK, Peek G, Firmin RK, Field DJ, et al. (2006). Developmental outcome in newborn infants treated for acute respiratory failure with extracorporeal membrane oxygenation: present experience. Arch Dis Child Fetal Neonatal Ed. 91:F21–F25.
- Singh Y, Lakshminrusimha S. (2021). Pathophysiology and Management of Persistent Pulmonary Hypertension of the Newborn. Clin Perinatol. 48(3):595-618.
- de Boode WP, Singh Y, Molnar Z, Schubert U, Savoia M, Sehgal A, et al. (2018). Application of Neonatologist Performed Echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr Res. 84 (Suppl 1):68–77.
- Singh Y, Katheria A, Tissot C. (2018). Functional Echocardiography in the Neonatal Intensive Care Unit. Indian Pediatr. 55(5):417-424.
 Abstract
Abstract  PDF
PDF