Past Issues
Pharmacological Management of Low Blood Flow State in Less than 28 weeks Neonates
Femi Adeniyi ST6 Paediatrics1*, Kunle Oyedokun ST7 Paediatrics2, Adeniyi Ajiboye ST43, Sanjeev Rath Neonatal Consultant4
Arrowe Park Hospital, Wirral University Teaching Hospital, Arrowe Park Road, Birkenhead, Wirral, CH49 5PE, UK
*Corresponding Author: Femi Adeniyi, Arrowe Park Hospital, Wirral University Teaching Hospital, Arrowe Park Road, Birkenhead, Wirral, CH49 5PE, UK, E-mail: [email protected].
Citation: Adeniyi F, et al. (2021). Pharmacological Management of Low Blood Flow State in Less than 28 weeks Neonates. Neonatal. 2(1):04.
Copyright: Adeniyi F, et al. ©2021.
Received: January 18, 2021
Published: June 29, 2021
BRIEF OVERVIEW
Low blood flow state is defined as insufficient cardiac output to maintain adequate cellular metabolism at the organ level. Low blood flow state can be measured by reduced organ perfusion such as reduced superior vena cava flow [1,2] or high resistance flow in superior mesenteric doppler [3]. According to Melitin J, et al. (2008) [4], capillary refill time of greater than 4 seconds and serum lactate greater than 4mmol/litre has 97% sensitivity of identifying low blood flow state.
In the presence of above markers of low blood flow state, the blood pressure may be normal or high in the first 48 hours of life due to high systemic resistance [5]. Therefore, high or normal blood pressure should be interpreted with great caution.
Literatures was sourced through health care database advanced search using articles and journals from Medline, PubMed and Embase in designing evidenced based guidance on pharmacological treatment of low blood flow state (Appendix 1).
KEYWORDS: Preterm; Prematurity; Extreme Prematurity AND Hypotension; Low blood pressure AND Inotropes; Therapeutics; Pharmacological
MANAGEMENT OF LOW BLOOD FLOW STATES
According to Pladys P, et al. (1999) [6] and Kluchow M, et al.(1996) [7], the hemodynamically significant shunt and low index of resistance to left ventricular output in early postnatal life were emphasised as a cause of low blood flow state in extreme preterm neonates. Hence, the important role of predominant vasopressors (dopamine, noradrenaline, adrenaline, vasopressin).
However, Saleemi MSH, et al. (2014) [8] explained that there is no significant improvement in myocardial function in the first four weeks of life in eight extreme preterm neonates. The use of inotropes such as dobutamine and milrinone as first line drug may be considered in extreme preterm neonates with low blood flow state.
It is important to note that pharmacological treatment of low blood state in extreme preterm neonates during the first 3 days of life is linked to increase risk of intraventricular haemorrhage or mortality [9]. Hence cautious use of pharmacological treatment is advised.
In view of the overlapping causes of low blood flow state and the possible adverse consequences of pharmacological treatment in preterm neonate, serial echocardiography is necessary in deciding choice of pharmacological treatment [10].
The international consensus on new-born resuscitation against the routine use of fluid bolus [11].
DOPAMINE
According Sassano-Higgins S, et al. (2011) [12], dopamine is more effective than dobutamine, colloids, and hydrocortisone, in increasing blood pressure. Although, dopamine can cause significant increase in kidney function at a lower dose without any effect on blood pressure or heart rate [13]. It is therefore important to titrate the dopamine dose to achieve the desired effect. The limitations of the literature review by Sassano-Higgins S, et al. (2011) [12] is the variation in the studies used which included prospective case series report, blinded and non-blinded randomized control trial.
According to Munro MJ, et al. (2004) & Valverde E, et al. (2005) [14,15] reported similar effects of dopamine versus low dose adrenaline on increasing the blood pressure and cerebral perfusion in hypotensive preterm neonates. It is worth emphasizing the small numbers involved in the above studies and result should be interpreted with caution.
It is important to note that dopamine at higher doses of greater than 10microgram/kg/minutes may result in impaired cerebral perfusion [16,17]. In the above studies, the cohort that received Dopamine was significantly unwell compared to the control group, making it difficult to attribute the impaired cerebral autoregulation to Dopamine alone.
Dopamine remains the first choice of vasopressor in preterm neonate with low blood flow state [18]. The use of another concomitant vasopressor is advised when approaching the dopamine dose of ten microgram/kg/minutes.
DOBUTAMINE
Dobutamine is the second most used drug in patients unresponsive to dopamine [19]. According to Robel-Tillig E, et al. (2007) [20], dobutamine's use at ten microgram/kg/minute may cause increase cardiac output and superior mesenteric, renal artery, and cerebral blood flow. However, the authors identified 8-10 hours delay in cerebral blood flow, superior mesenteric artery, renal artery flow following dobutamine administration. In contrast, Hentschel R, et al. (1995) [21] emphasize similar effect of dobutamine and dopamine at doses of 10microgram/kg/minutes on superior mesenteric artery flow rate. It should be noted that there is a dearth of evidence on dobutamine's impact at higher doses on superior mesenteric, renal arteries, and cerebral blood flow [22]. It is worth emphasizing the need to assess the superior mesenteric flow when using dobutamine and consider adding another pharmacological agent to counteract the possible delay in gut perfusion.
Dobutamine use is advised in extreme preterm neonates with myocardial dysfunction secondary to pulmonary hypertension and in post ligation cardiac syndrome [23].
NORADRENALINE
Noradrenaline is tolerated safely in preterms less than 32weeks treated for sepsis and pulmonary hypertension [24]. The authors stated that noradrenaline at dose of 0.5mcg/kg/minutes caused the desired blood pressure effect in within 1 hour. The measured outcome in the study is blood pressure effect rather than low blood flow state. Hence, the study should be interpreted with caution.
According to Jaillard S, et al. (2004) [25] in their randomized case-control experimental study in fetal lambs, noradrenaline improves post-natal adaptation in pulmonary hypertension by increasing systemic vascular pressure and increasing pulmonary blood flow. Furthermore, Tourneux P, et al. (2008) [26] added that noradrenaline may cause improvement in pulmonary function by increasing pulmonary/ systemic artery pressure ratio and improved cardiac function.
Noradrenaline use as choice of vasopressor is suggested in hypotensive preterm neonates with pulmonary hypertension.
ADRENALINE
The use of adrenaline in new-born resuscitation for the return of spontaneous circulation is a recognized practice [27]. According to Valverde E, et al. (2006) [15] prospective observation study concluded that a low to moderate dose of adrenaline is as effective as a low to moderate dopamine dose in the treatment of hypotension in low-birth weight neonates. It is essential to emphasize the complication associated with adrenaline, such as high heart rate, high lactate, lower base excess and lower bicarbonates.
Furthermore, Paradisis M, Osborn DA (2004) [28] Cochrane review suggested that there is insufficient evidence on the use of adrenaline as first line in preterm neonates with low blood flow state.
The consensus is on the use of Adrenaline when there is no spontaneous return of adequate circulation.
CORTICOSTEROID
Corticosteroids are known for treating hypotension resistant to fluid expansion and two vasopressors [29]. The routine use of hydrocortisone as 1st line in the treatment of hypotension is frowned upon due to complications of sepsis, gut perforation [30]. According to Bouchier D, et al. (1997) [31], hydrocortisone might be as effective as dopamine in treating hypotension. This agreed with Bosante F, et al. (2007) [32] that the prophylactic use of low dose hydrocortisone (Appendix 4) reduced the use of vasopressors. On the other hand, Ng PC, et al. (2006) [33] concluded that a hydrocortisone's stress dose (Appendix 4) effectively treated refractory hypotension.
According to Shaffer ML, et al. (2019) [34], who further studied the long-term effects of low dose hydrocortisone prophylaxis in extremely preterm neonates concluded no adverse effects for either death or 2-year neurodevelopmental delay. Conversely, Subhedar NV, (2011) [35] in their Cochrane systematic review, much-discouraged routine uses of dexamethasone in preterm hypotension based on lack of long-term safety profile.
Hydrocortisone remains a safe drug in the treatment of refractory hypotension. The use of baseline cortisol in deciding the need for therapy is encouraged [36].
MILRINONE
Milrinone prophylaxis does not prevent low blood flow states in high-risk preterm neonates, according to Paradisis M, Osborn DA (2004) [28]. In addition, Halliday M, et al. (2017) [37] discouraged the prophylactic use of milrinone post-patent ductus arteriosus ligation with emphasis that prophylactic milrinone has no impact on immediate cardiovascular or long-term outcomes. Therefore, the prophylactic use of Milrinone is not suggested in everyday practice.
In contrast, a case series report suggested that milrinone is a useful therapy in infants with echocardiography findings of pulmonary hypertension or right heart dysfunction [38]. Furthermore, Jain A, et al. (2012) [39], demonstrated that administration of milrinone to neonates with low cardiac output before PDA ligation might lead to improved postoperative stability. The use of milrinone is encouraged as second choice inotropes when there is suboptimal response to dobutamine in preterm neonates with myocardial dysfunction and pulmonary hypertension.
VASOPRESSIN
According to Mohamed AA, et al. (2020) [40] and Bidegain M, et al. (2010) [41] complications such as liver necrosis, splanchnic hypoperfusion and high mortality associated with use of vasopressin in extreme preterm neonates. In contrast, Ni M, et al. (2017) [42], Rios DR, and Kaiser JR (2015) [43] reported safe use of vasopressin in preterm neonates. The side effect such as raised lactate and hyponatremia were not stated by Meyers S, et al. (2006) [44] which also support the use of vasopressin in extreme preterm neonates resistant to catecholamine treatment.
There is insufficient evidence to recommend everyday use of vasopressin in neonates for treatment of low blood flow state [45]. The use is reserved for very severe low blood flow state that is resistant to catecholamine treatment [46].
LIMITATIONS
The quality of evidence is sub-optimal, the majority are observational studies with high heterogenicity and less representation of less than 24weeks, hence creating a high confounding factor. In addition, there is variability between different centres in diagnosing low blood flow state, some of the studies used low mean or systolic blood pressure to determine the effect of treatment.
CONCLUSION
The pharmacological treatment of low blood flow state should be guided by thorough clinical assessment (Appendix 2). The prophylaxis or stress dose hydrocortisone treatment of low flow state is gaining grounds mainly when there is evidence of adrenal insufficiency (Appendix 3).
The choice and titration of pharmacological treatment should be guided by functional echocardiography. The use of Dobutamine as first line treatment is advised when myocardia dysfunction on echocardiography is noted.
Milrinone use is reserved for extreme preterm neonates with myocardia dysfunction prior to patent ductus arteriosus ligation. Dopamine and noradrenaline remains the commonly used first- and second-line vasopressor respectively in the management of low blood flow state secondary to poor vasomotor resistance.
CONSENT
Not required.
REFERENCES
- Osborn DA, Evans N, Kluckow M, Bowen JR, Rieger I. (2007). Low Superior Vena Cava Flow and Effect of Inotropes on Neurodevelopment to 3 Years in Preterm Infants. Pediatrics. 120:372-380.
- Evans N, Kluckow M, Simmons M, Osborn D. (2002). Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 87:F181-F184.
- Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GCS, Kempley ST. (2006). Doppler Flow Velocimetry in the Superior Mesenteric Artery on the First Day of Life in Preterm Infants and the Risk of Neonatal Necrotizing Enterocolitis. Pediatrics. 118:1999-2003.
- Miletin J, Pichova K, Dempsey EM. (2008). Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. European Journal of Pediatrics. 168:809.
- Groves AM, Kuschel CA, Knight DB, Skinner JR. (2008). Relationship between blood pressure and blood flow in newborn preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 93:F29-F32.
- Pladys P, Wodey E, Beuchée A, Branger B, Bétrémieux P. (1999). Left ventricle output and mean arterial blood pressure in preterm infants during the 1st day of life. European Journal of Pediatrics. 158:817-824.
- Kluckow M, Evans N. (1996). Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. The Journal of Pediatrics. 129:506-512.
- Saleemi MSH, El-Khuffash A, Franklin O, David Corcoran J. (2014). Serial changes in myocardial function in preterm infants over a four week period: the effect of gestational age at birth. Early Human Development. 90:349-352.
- Aziz AZA, Thomas S, Murthy P, Rabi Y, Soraisham A, et al. (2020). Early inotropes use is associated with higher risk of death and/or severe brain injury in extremely premature infants. The Journal of Maternal-Fetal & Neonatal Medicine. 33:2751-2758.
- Pawale D, Murki S, Kulkarni D, Vardhelli V, Sharma D, et al. (2020). Echocardiographic assessment of hemodynamic changes in preterm neonates with shock: a prospective pragmatic cohort study. European Journal of Pediatrics. 179:1893-1899.
- Wyllie J, Perlman JM, Kattwinkel J, Atkins DL, Chameides L, et al. (2010). Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 81:e260-e287.
- Sassano-Higgins S, Friedlich P, Seri I. (2011). A meta-analysis of dopamine use in hypotensive preterm infants: blood pressure and cerebral hemodynamics. Journal of Perinatology. 31:647-655.
- Lynch SK, Lemley KV, Polak MJ. (2003). The effect of dopamine on glomerular filtration rate in normotensive, oliguric premature neonates. Pediatric Nephrology. 18:649-652.
- Munro MJ, Walker AM, Barfield CP. (2004). Hypotensive Extremely Low Birth Weight Infants Have Reduced Cerebral Blood Flow. Pediatrics. 114:1591-1596.
- Valverde E, Pellicer A, Madero R, Elorza D, Quero J, et al. (2006). Dopamine Versus Epinephrine for Cardiovascular Support in Low Birth Weight Infants: Analysis of Systemic Effects and Neonatal Clinical Outcomes. Pediatrics. 117:e1213-e1222.
- Solanki NS, Hoffman SB. (2020). Association between dopamine and cerebral autoregulation in preterm neonates. Pediatric Research. 88:618-622.
- Eriksen VR, Hahn GH, Greisen G. (2014). Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatrica. 103:1221-1226.
- Bhayat SI, Gowda HMS, Eisenhut M. (2016). Should dopamine be the first line inotrope in the treatment of neonatal hypotension? Review of the evidence. World journal of clinical paediatrics. 5:212-222.
- Dasgupta SJ, Gill AB. (2003). Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Archives of Disease in Childhood - Fetal and Neonatal Edition. 88:F450-F454.
- Robel-Tillig E, Knupfer M, Pulzer F, Vogtmann C. (2007). Cardiovascular impact of dobutamine in neonates with myocardial dysfunction. Early Human Development. 83:307-312.
- Hentschel R, Hensel D, Brune T, Rabe H, Jorch G. (1995). Impact on Blood Pressure and Intestinal Pertusion of Dobutamine or Dopamine in Hypotensive Preterm Infants. Neonatology. 68:318-324.
- Osborn DA, Paradisis M, Evans NJ. (2007). The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database of Systematic Reviews.
- Korkmaz L, Ozdemir A, Pamukcu O, Günes T, Ozturk MA. (2020). Which Inotropic Drug, Dobutamine or Milrinone, Is Clinically More Effective in the Treatment of Postligation Cardiac Syndrome in Preterm Infants? Am J Perinatol.
- Rowcliff K, De Waal K, Mohamed AL, Chaudhari T. (2016). Noradrenaline in preterm infants with cardiovascular compromise. European Journal of Pediatrics. 175:1967-1973.
- Jaillard S, Elbaz F, Bresson-Just S, Riou Y, Houfflin-Debarge V, et al. (2004). Pulmonary vasodilator effects of norepinephrine during the development of chronic pulmonary hypertension in neonatal lambs. BJA: British Journal of Anaesthesia. 93:818-824.
- Tourneux P, Rakza T, Bouissou A, Krim G, Storme L. (2008). Pulmonary Circulatory Effects of Norepinephrine in Newborn Infants with Persistent Pulmonary Hypertension. The Journal of Pediatrics. 153:345-349.
- Antonucci R, Antonucci L, Locci C, Porcella A, Cuzzolin L. (2018). Current Challenges in Neonatal Resuscitation: What is the Role of Adrenaline? Pediatric Drugs. 20:417-428.
- Paradisis M, Osborn DA. (2004). Adrenaline for prevention of morbidity and mortality in preterm infants with cardiovascular compromise. Cochrane Database of Systematic Reviews. (1):CD003958.
- Baker CFW, Barks JDE, Engmann C, Vazquez DM, Neal CR, et al. (2008). Hydrocortisone administration for the treatment of refractory hypotension in critically ill newborns. Journal of Perinatology. 28:412-419.
- SERI I. (2006). Hydrocortisone and Vasopressor-Resistant Shock in Preterm Neonates. Pediatrics. 117:516-518.
- Bourchier D, Weston PJ. (1997). Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Archives of Disease in Childhood - Fetal and Neonatal Edition. 76:F174-F178.
- Bonsante F, Latorre G, Iacobelli S, Forziati V, Laforgia N, et al. (2007). Early Low-Dose Hydrocortisone in Very Preterm Infants: A Randomized, Placebo-Controlled Trial. Neonatology. 91:217-221.
- Ng PC, Lee CH, Bnur FL, Chan IHS, Lee AWY, et al. (2006). A Double-Blind, Randomized, Controlled Study of a “Stress Dose” of Hydrocortisone for Rescue Treatment of Refractory Hypotension in Preterm Infants. Pediatrics. 117:367-375.
- Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, et al. (2019). Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. The Journal of Pediatrics. 207:136-142.e5.
- Ibrahim H, Sinha IP, Subhedar NV. (2011). Corticosteroids for treating hypotension in preterm infants. Cochrane Database of Systematic Reviews. 2011(12):CD003662.
- PEEPLES ES. (2017). An evaluation of hydrocortisone dosing for neonatal refractory hypotension. Journal of Perinatology. 37:943-946.
- Halliday M, Kavarana M, Ebeling M, Kiger J. (2017). Milrinone use for hemodynamic instability in patent ductus arteriosus ligation. The Journal of Maternal-Fetal& Neonatal Medicine. 30:529-533.
- James AT, Bee C, Corcoran JD, Mcnamara PJ, Franklin O, et al. (2015). Treatment of premature infants with pulmonary hypertension and right ventricular dysfunction with milrinone: a case series. Journal of Perinatology. 35:268-273.
- Jain A, Sahni M, El-Khuffash A, Khadawardi E, Sehgal A, et al. (2012). Use of Targeted Neonatal Echocardiography to Prevent Postoperative Cardiorespiratory Instability after Patent Ductus Arteriosus Ligation. The Journal of Pediatrics. 160:584-589.e1.
- Mohamed AA, Louis D, Surak A, Weisz DE, Mcnamara PJ, et al. (2020). Vasopressin for refractory persistent pulmonary hypertension of the newborn in preterm neonates – a case series. The Journal of Maternal-Fetal & Neonatal Medicine. 1-9.
- Bidegain M, Greenberg R, Simmons C, Dang C, Cotten CM, et al. (2010). Vasopressin for Refractory Hypotension in Extremely Low Birth Weight Infants. The Journal of Pediatrics. 157:502-504.
- Ni M, Kaiser JR, Moffett BS, Rhee CJ, Placencia J, et al. (2017). Use of Vasopressin in Neonatal Intensive Care Unit Patients with Hypotension. The Journal of Pediatric Pharmacology and Therapeutics. 22:430-435.
- Rios DR, Kaiser JR. (2015). Vasopressin versus Dopamine for Treatment of Hypotension in Extremely Low Birth Weight Infants: A Randomized, Blinded Pilot Study. The Journal of Pediatrics. 166:850-855.
- Meyer S, Gottschling S, Baghai A, Wurm D, Gortner L. (2006). Arginine-vasopressin in catecholamine-refractory septic versus non-septic shock in extremely low birth weight infants with acute renal injury. Critical Care. 10:R71.
- Shivanna B, Rios D, Rossano J, Fernandes CJ, Pammi M. (2013). Vasopressin and its analogues for the treatment of refractory hypotension in neonates. Cochrane Database of Systematic Reviews.
- Ikegami H, Funato M, Tamai H, Wada H, Nabetani M, et al. (2010). Low-dose vasopressin infusion therapy for refractory hypotension in ELBW infants. Pediatrics International. 52:368-373.
APPENDIX 1 Literatures were sourced through health care database advanced search using articles and journals from Embase, Medline and PubMed. Keywords used are: Preterm OR Prematurity OR Extreme Prematurity AND Hypotension OR Low blood pressure AND Inotropes OR Therapeutics OR Pharmacological. 210 articles were obtained between 1995 to 2020. 79 excluded due to duplication. 66 of the remaining 131 were published in the last 10years. The pharmacological choice for the management of low blood flow states was obtained from publications in the last 10years.
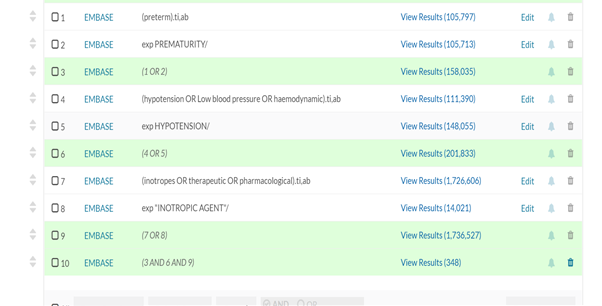
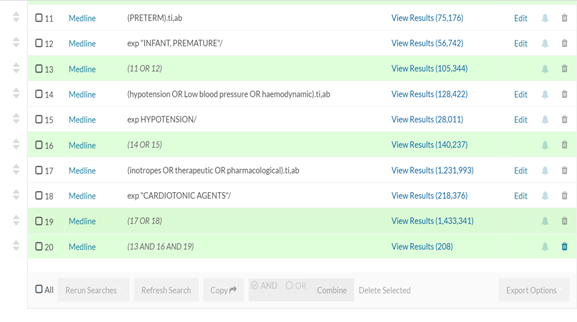
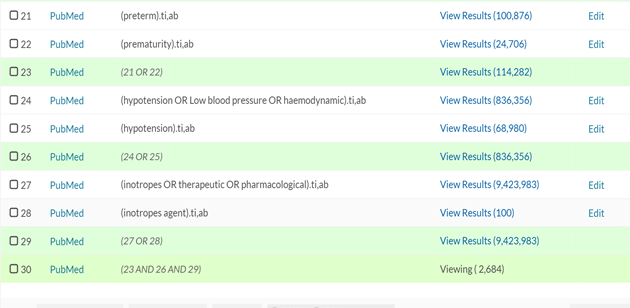
APPENDIX 2
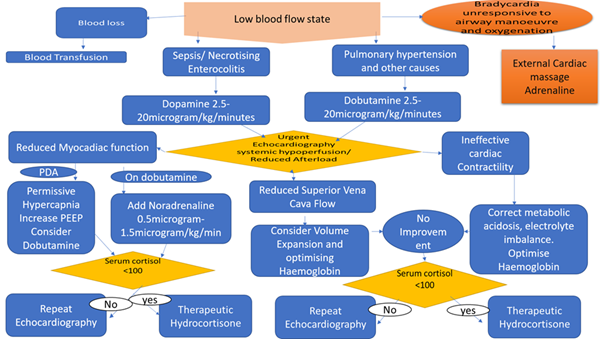
APPENDIX 3
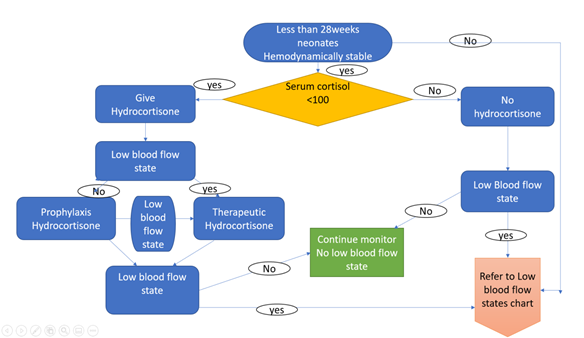
APPENDIX 4
| Hydrocortisone | Dosage |
| Prophylaxis | 0.5 mg/kg/12 hourly for 9 days, then 0.5 mg/kg/24 hourly for 3 days |
| Therapeutic stress dose | 1mg/kg eight hourly for five days |
Copyright: Adeniyi F, et al. ©2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Adeniyi F, et al. (2021). Pharmacological Management of Low Blood Flow State in Less than 28 weeks Neonates. Neonatal. 2(1):04.
 Abstract
Abstract  PDF
PDF